Ecological aspects of the distribution of different autotrophic CO2 fixation pathways
- PMID: 21216907
- PMCID: PMC3067309
- DOI: 10.1128/AEM.02473-10
Ecological aspects of the distribution of different autotrophic CO2 fixation pathways
Abstract
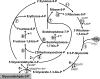
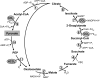
Autotrophic CO(2) fixation represents the most important biosynthetic process in biology. Besides the well-known Calvin-Benson cycle, five other totally different autotrophic mechanisms are known today. This minireview discusses the factors determining their distribution. As will be made clear, the observed diversity reflects the variety of the organisms and the ecological niches existing in nature.
Figures

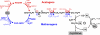


References
-
- Anderson, L. E. 1971. Chloroplast and cytoplasmic enzymes. II. Pea leaf triose phosphate isomerases. Biochim. Biophys. Acta 235:237-244. - PubMed
-
- Aoshima, M. 2007. Novel enzyme reactions related to the citric acid cycle: phylogenetic/functional implications and biotechnological applications. Appl. Microbiol. Biotechnol. 75:249-255. - PubMed
-
- Aoshima, M., M. Ishii, and Y. Igarashi. 2004. A novel enzyme, citryl-CoA synthetase, catalysing the first step of the citrate cleavage reaction in Hydrogenobacter thermophilus TK-6. Mol. Microbiol. 52:751-761. - PubMed
-
- Aoshima, M., M. Ishii, and Y. Igarashi. 2004. A novel enzyme, citryl-CoA lyase, catalysing the second step of the citrate cleavage reaction in Hydrogenobacter thermophilus TK-6. Mol. Microbiol. 52:763-770. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

