The nucleus-encoded trans-acting factor MCA1 plays a critical role in the regulation of cytochrome f synthesis in Chlamydomonas chloroplasts
- PMID: 21216944
- PMCID: PMC3051260
- DOI: 10.1105/tpc.110.078170
The nucleus-encoded trans-acting factor MCA1 plays a critical role in the regulation of cytochrome f synthesis in Chlamydomonas chloroplasts
Abstract
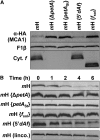
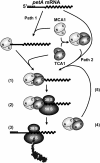
Organelle gene expression is characterized by nucleus-encoded trans-acting factors that control posttranscriptional steps in a gene-specific manner. As a typical example, in Chlamydomonas reinhardtii, expression of the chloroplast petA gene encoding cytochrome f, a major subunit of the cytochrome b(6)f complex, depends on MCA1 and TCA1, required for the accumulation and translation of the petA mRNA. Here, we show that these two proteins associate in high molecular mass complexes that also contain the petA mRNA. We demonstrate that MCA1 is degraded upon interaction with unassembled cytochrome f that transiently accumulates during the biogenesis of the cytochrome b(6)f complex. Strikingly, this interaction relies on the very same residues that form the repressor motif involved in the Control by Epistasy of cytochrome f Synthesis (CES), a negative feedback mechanism that downregulates cytochrome f synthesis when its assembly within the cytochrome b(6)f complex is compromised. Based on these new findings, we present a revised picture for the CES regulation of petA mRNA translation that involves proteolysis of the translation enhancer MCA1, triggered by its interaction with unassembled cytochrome f.
Figures








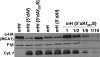

References
-
- Ackerman S.H., Tzagoloff A. (2005). Function, structure, and biogenesis of mitochondrial ATP synthase. Prog. Nucleic Acid Res. Mol. Biol. 80: 95–133 - PubMed
-
- Anderson S., et al. (1981). Sequence and organization of the human mitochondrial genome. Nature 290: 457–465 - PubMed
-
- Balczun C., Bunse A., Hahn D., Bennoun P., Nickelsen J., Kück U. (2005). Two adjacent nuclear genes are required for functional complementation of a chloroplast trans-splicing mutant from Chlamydomonas reinhardtii. Plant J. 43: 636–648 - PubMed
-
- Barkan A., Goldschmidt-Clermont M. (2000). Participation of nuclear genes in chloroplast gene expression. Biochimie 82: 559–572 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

