The PP2A regulatory subunit Tap46, a component of the TOR signaling pathway, modulates growth and metabolism in plants
- PMID: 21216945
- PMCID: PMC3051261
- DOI: 10.1105/tpc.110.074005
The PP2A regulatory subunit Tap46, a component of the TOR signaling pathway, modulates growth and metabolism in plants
Abstract
Tap42/α4, a regulatory subunit of protein phosphatase 2A, is a downstream effector of the target of rapamycin (TOR) protein kinase, which regulates cell growth in coordination with nutrient and environmental conditions in yeast and mammals. In this study, we characterized the functions and phosphatase regulation of plant Tap46. Depletion of Tap46 resulted in growth arrest and acute plant death with morphological markers of programmed cell death. Tap46 interacted with PP2A and PP2A-like phosphatases PP4 and PP6. Tap46 silencing modulated cellular PP2A activities in a time-dependent fashion similar to TOR silencing. Immunoprecipitated full-length and deletion forms of Arabidopsis thaliana TOR phosphorylated recombinant Tap46 protein in vitro, supporting a functional link between Tap46 and TOR. Tap46 depletion reproduced the signature phenotypes of TOR inactivation, such as dramatic repression of global translation and activation of autophagy and nitrogen mobilization, indicating that Tap46 may act as a positive effector of TOR signaling in controlling those processes. Additionally, Tap46 silencing in tobacco (Nicotiana tabacum) BY-2 cells caused chromatin bridge formation at anaphase, indicating its role in sister chromatid segregation. These findings suggest that Tap46, in conjunction with associated phosphatases, plays an essential role in plant growth and development as a component of the TOR signaling pathway.
Figures



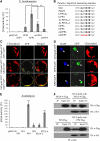



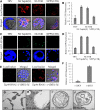
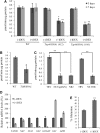
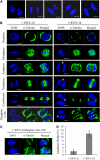

References
-
- Ahn J.W., Kim M., Lim J.H., Kim G.T., Pai H.S. (2004). Phytocalpain controls the proliferation and differentiation fates of cells in plant organ development. Plant J. 38: 969–981 - PubMed
-
- Aoyama T., Chua N.-H. (1997). A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J. 11: 605–612 - PubMed
-
- Azevedo C., Sadanandom A., Kitagawa K., Freialdenhoven A., Shirasu K., Schulze-Lefert P. (2002). The RAR1 interactor SGT1, an essential component of R gene-triggered disease resistance. Science 295: 2073–2076 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

