Human T-lymphotropic virus type 1 p30 interacts with REGgamma and modulates ATM (ataxia telangiectasia mutated) to promote cell survival
- PMID: 21216954
- PMCID: PMC3045020
- DOI: 10.1074/jbc.M110.176354
Human T-lymphotropic virus type 1 p30 interacts with REGgamma and modulates ATM (ataxia telangiectasia mutated) to promote cell survival
Abstract
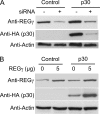
Human T-lymphotropic virus type 1 (HTLV-1) is a causative agent of adult T cell leukemia/lymphoma and a variety of inflammatory disorders. HTLV-1 encodes a nuclear localizing protein, p30, that selectively alters viral and cellular gene expression, activates G(2)-M cell cycle checkpoints, and is essential for viral spread. Here, we used immunoprecipitation and affinity pulldown of ectopically expressed p30 coupled with mass spectrometry to identify cellular binding partners of p30. Our data indicate that p30 specifically binds to cellular ATM (ataxia telangiectasia mutated) and REGγ (a nuclear 20 S proteasome activator). Under conditions of genotoxic stress, p30 expression was associated with reduced levels of ATM and increased cell survival. Knockdown or overexpression of REGγ paralleled p30 expression, suggesting an unexpected enhancement of p30 expression in the presence of REGγ. Finally, size exclusion chromatography revealed the presence of p30 in a high molecular mass complex along with ATM and REGγ. On the basis of our findings, we propose that HTLV-1 p30 interacts with ATM and REGγ to increase viral spread by facilitating cell survival.
Figures





References
-
- Yoshida M. (2005) Oncogene 24, 5931–5937 - PubMed
-
- Lairmore M., Franchini G. (2007) in Fields Virology (Knipe D. M. ed) pp. 2071–2105, Wolters Kluwer/Lippincott Williams and Wilkins, Philadelphia, PA
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

