Inhibition of complement alternative pathway suppresses experimental autoimmune anterior uveitis by modulating T cell responses
- PMID: 21216963
- PMCID: PMC3048731
- DOI: 10.1074/jbc.M110.197616
Inhibition of complement alternative pathway suppresses experimental autoimmune anterior uveitis by modulating T cell responses
Abstract
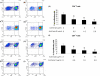
The objective of the current study was to delineate the pathway of complement activation that is crucial for the induction of experimental autoimmune anterior uveitis (EAAU). We studied the development of EAAU in melanin-associated antigen (MAA)-sensitized Lewis rats treated with antibody against C4 or factor B. Control animals received isotype IgG control. Antibody against C4 had no effect on EAAU, and all of the animals developed EAAU similar to those injected with control IgG. In contrast, EAAU was completely inhibited in all MAA-sensitized Lewis rats injected with factor B antibody. Treatment with anti-factor B antibody resulted in suppression of ocular complement activation. Adoptive transfer of T lymphocytes harvested from draining lymph nodes of donor animals treated with anti-factor B did not transfer EAAU to naïve syngenic rats. Anti-factor B antibody inhibited the ability of MAA-specific CD4(+) T cells to proliferate (in vitro) in response to MAA in a dose-dependent manner. Level of TNF-α and IFN-γ decreased in the presence of anti-factor B. Collectively, our results provide the novel finding that complement activation via the alternative pathway contributes to intraocular inflammation in EAAU, and anti-factor B-mediated inhibition of EAAU is due to diminished antigen-specific CD4(+) T cell responses to MAA. Our findings explain the interactions between the complement system and T cells that are critical for the induction of EAAU and may lead to the development of therapy for idiopathic anterior uveitis based on selective blockade of the alternative pathway.
Figures

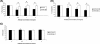


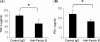
Similar articles
-
Induction of experimental autoimmune anterior uveitis by a self-antigen: melanin complex without adjuvant.Invest Ophthalmol Vis Sci. 1997 Sep;38(10):2171-5. Invest Ophthalmol Vis Sci. 1997. PMID: 9331282
-
Inhibitory role of transforming growth factor β2 in experimental autoimmune anterior uveitis.Graefes Arch Clin Exp Ophthalmol. 2019 May;257(5):953-960. doi: 10.1007/s00417-019-04255-9. Epub 2019 Feb 5. Graefes Arch Clin Exp Ophthalmol. 2019. PMID: 30719689
-
Immunohistochemical studies on melanin associated antigen (MAA) induced experimental autoimmune anterior uveitis (EAAU).Curr Eye Res. 1995 Aug;14(8):703-10. doi: 10.3109/02713689508998498. Curr Eye Res. 1995. PMID: 8529406
-
Experimental melanin-induced uveitis: experimental model of human acute anterior uveitis.Ophthalmic Res. 2008;40(3-4):136-40. doi: 10.1159/000119864. Epub 2008 Apr 18. Ophthalmic Res. 2008. PMID: 18421227 Review.
-
[Intraocular inflammation and homeostasis of the eye].Nippon Ganka Gakkai Zasshi. 2009 Mar;113(3):344-77; discussion 378. Nippon Ganka Gakkai Zasshi. 2009. PMID: 19348183 Review. Japanese.
Cited by
-
Complement anaphylatoxin receptors C3aR and C5aR are required in the pathogenesis of experimental autoimmune uveitis.J Leukoc Biol. 2016 Mar;99(3):447-54. doi: 10.1189/jlb.3A0415-157R. Epub 2015 Sep 22. J Leukoc Biol. 2016. PMID: 26394814 Free PMC article.
-
Complement Component C4 Regulates the Development of Experimental Autoimmune Uveitis through a T Cell-Intrinsic Mechanism.Front Immunol. 2017 Sep 11;8:1116. doi: 10.3389/fimmu.2017.01116. eCollection 2017. Front Immunol. 2017. PMID: 28955337 Free PMC article.
-
Chitosan oligosaccharides attenuate ocular inflammation in rats with experimental autoimmune anterior uveitis.Mediators Inflamm. 2014;2014:827847. doi: 10.1155/2014/827847. Epub 2014 Jul 24. Mediators Inflamm. 2014. PMID: 25147441 Free PMC article.
-
Targeting the Complement Alternative Pathway Permits Graft Versus Leukemia Activity while Preventing Graft Versus Host Disease.Clin Cancer Res. 2020 Jul 1;26(13):3481-3490. doi: 10.1158/1078-0432.CCR-19-1717. Epub 2020 Jan 9. Clin Cancer Res. 2020. PMID: 31919135 Free PMC article.
-
Autoimmune uveitis: clinical, pathogenetic, and therapeutic features.Clin Exp Med. 2016 May;16(2):125-36. doi: 10.1007/s10238-015-0345-6. Epub 2015 Mar 28. Clin Exp Med. 2016. PMID: 25820692 Review.
References
-
- Gritz D. C., Wong I. G. (2004) Ophthalmology 111, 491–500 - PubMed
-
- Bora N. S., Kaplan H. J. (2007) Chem. Immunol. Allergy 92, 213–220 - PubMed
-
- Bloch-Michel E., Nussenblatt R. B. (1987) Am. J. Ophthalmol. 103, 234–235 - PubMed
-
- Broekhuyse R. M., Kuhlmann E. D., Winkens H. J., Van Vugt A. H. (1991) Exp. Eye Res. 52, 465–474 - PubMed
-
- Bora N. S., Kim M. C., Kabeer N. H., Simpson S. C., Tandhasetti M. T., Cirrito T. P., Kaplan A. D., Kaplan H. J. (1995) Invest. Ophthalmol. Vis. Sci. 36, 1056–1066 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

