Earliest innate immune responses require macrophage RelA during pneumococcal pneumonia
- PMID: 21216972
- PMCID: PMC3175578
- DOI: 10.1165/rcmb.2010-0210OC
Earliest innate immune responses require macrophage RelA during pneumococcal pneumonia
Abstract
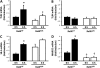
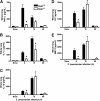
NF-κB regulates cytokine expression to initiate and control the innate immune response to lung infections. The NF-κB protein RelA is critical for pulmonary host defense during Streptococcus pneumoniae pneumonia, but the cell-specific roles of this transcription factor remain to be determined. We hypothesized that RelA in alveolar macrophages contributes to cytokine expression and host defense during pneumococcal pneumonia. To test this hypothesis, we compared mice lacking RelA exclusively in myeloid cells (RelA(Δ/Δ)) with littermate controls (RelA(F/F)). Alveolar macrophages from RelA(Δ/Δ) mice expressed no full-length RelA, demonstrating effective targeting. Alveolar macrophages from RelA(Δ/Δ) mice exhibited reduced, albeit detectable, proinflammatory cytokine responses to S. pneumoniae, compared with alveolar macrophages from RelA(F/F) mice. Concentrations of these cytokines in lung homogenates were diminished early after infection, indicating a significant contribution of macrophage RelA to the initial expression of cytokines in the lungs. However, the cytokine content in infected lungs was equivalent by 15 hours. Neutrophil recruitment during S. pneumoniae pneumonia reflected a delayed onset in RelA(Δ/Δ) mice, followed by similar rates of accumulation. Bacterial clearance was eventually effective in both genotypes, but began later in RelA(Δ/Δ) mice. Thus, during pneumococcal pneumonia, only the earliest induction of the cytokines measured depended on transcription by RelA in myeloid cells, and this transcriptional activity contributed to effective immunity.
Figures




References
-
- Guest JF, Morris A. Community-acquired pneumonia: the annual cost to the National Health Service in the UK. Eur Respir J 1997;10:1530–1534 - PubMed
-
- Ruiz M, Ewig S, Torres A, Arancibia F, Marco F, Mensa J, Sanchez M, Martinez JA. Severe community-acquired pneumonia: risk factors and follow-up epidemiology. Am J Respir Crit Care Med 1999;160:923–929 - PubMed
-
- Garvy BA, Harmsen AG. The importance of neutrophils in resistance to pneumococcal pneumonia in adult and neonatal mice. Inflammation 1996;20:499–512 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

