Ribose 2'-O-methylation provides a molecular signature for the distinction of self and non-self mRNA dependent on the RNA sensor Mda5
- PMID: 21217758
- PMCID: PMC3182538
- DOI: 10.1038/ni.1979
Ribose 2'-O-methylation provides a molecular signature for the distinction of self and non-self mRNA dependent on the RNA sensor Mda5
Abstract
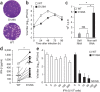
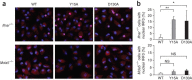
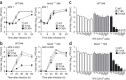
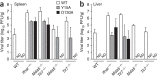
The 5' cap structures of higher eukaryote mRNAs have ribose 2'-O-methylation. Likewise, many viruses that replicate in the cytoplasm of eukaryotes have evolved 2'-O-methyltransferases to autonomously modify their mRNAs. However, a defined biological role for 2'-O-methylation of mRNA remains elusive. Here we show that 2'-O-methylation of viral mRNA was critically involved in subverting the induction of type I interferon. We demonstrate that human and mouse coronavirus mutants lacking 2'-O-methyltransferase activity induced higher expression of type I interferon and were highly sensitive to type I interferon. Notably, the induction of type I interferon by viruses deficient in 2'-O-methyltransferase was dependent on the cytoplasmic RNA sensor Mda5. This link between Mda5-mediated sensing of viral RNA and 2'-O-methylation of mRNA suggests that RNA modifications such as 2'-O-methylation provide a molecular signature for the discrimination of self and non-self mRNA.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Comment in
-
2 methylate or not 2 methylate: viral evasion of the type I interferon response.Nat Immunol. 2011 Feb;12(2):114-5. doi: 10.1038/ni0211-114. Nat Immunol. 2011. PMID: 21245900 Free PMC article.
References
-
- Loo YM, Gale M., Jr. Viral regulation and evasion of the host response. Curr. Top. Microbiol. Immunol. 2007;316:295–313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

