The fungicide ciclopirox inhibits lymphatic endothelial cell tube formation by suppressing VEGFR-3-mediated ERK signaling pathway
- PMID: 21217783
- PMCID: PMC3088775
- DOI: 10.1038/onc.2010.590
The fungicide ciclopirox inhibits lymphatic endothelial cell tube formation by suppressing VEGFR-3-mediated ERK signaling pathway
Abstract
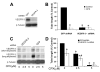
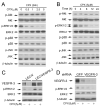
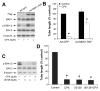
Ciclopirox olamine (CPX), an off-patent antifungal agent used to treat mycoses of skin and nails, has recently been demonstrated to be a potential anticancer agent. However, the underlying mechanism is not well understood. Here, for the first time, we show that CPX inhibited lymphangiogenesis in an in vitro model (tube formation). This effect was, in part, associated with inhibition of vascular endothelial growth factor receptor-3 (VEGFR-3) expression, as overexpression of VEGFR-3 conferred partial resistance to CPX inhibitory effect on tube formation in lymphatic endothelial cells (LECs), whereas downregulation of VEGFR-3 mimicked the effect of CPX, blocking the tube formation. Further study revealed that CPX did not alter mRNA level, but inhibited protein synthesis and promoted protein degradation of VEGFR-3. In addition, we found that CPX inhibited phosphorylation of the extracellular signal-related kinase 1/2 (ERK1/2), a downstream effector of VEGFR-3. Overexpression of VEGFR-3 attenuated CPX inhibition of ERK1/2 phosphorylation, whereas downregulation of VEGFR-3 inhibited ERK1/2 phosphorylation in LECs. Ectopic expression of constitutively active mitogen-activated protein kinase kinase 1 (MKK1) resulted in activation of ERK1/2 and partially prevented CPX inhibition of LEC tube formation. The results suggest that CPX inhibits LEC tube formation at least, in part, through inhibiting VEGFR-3-mediated ERK signaling pathway.
Figures



References
-
- Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell. 2002;1:219–227. - PubMed
-
- Ando T, Jordan P, Joh T, Wang Y, Jennings MH, Houghton J, et al. Isolation and characterization of a novel mouse lymphatic endothelial cell line: SV-LEC. Lymphat. Res. Biol. 2005;3:105–115. - PubMed
-
- Clement PM, Hanauske-Abel HM, Wolff EC, Kleinman HK, Park MH. The antifungal drug ciclopirox inhibits deoxyhypusine and proline hydroxylation, endothelial cell growth and angiogenesis in vitro. Int. J. Cancer. 2002;100:491–498. - PubMed
-
- Cueni LN, Detmar M. New insights into the molecular control of the lymphatic vascular system and its role in disease. J. Invest. Dermatol. 2006;126:2167–2177. - PubMed
-
- Dittmar W, Lohaus G. HOE 296, a new antimycotic compound with a broad antimicrobial spectrum. Laboratory results. Arzneimittelforschung. 1973;23:670–674. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

