The Drosophila FoxA ortholog Fork head regulates growth and gene expression downstream of Target of rapamycin
- PMID: 21217822
- PMCID: PMC3013099
- DOI: 10.1371/journal.pone.0015171
The Drosophila FoxA ortholog Fork head regulates growth and gene expression downstream of Target of rapamycin
Abstract
Forkhead transcription factors of the FoxO subfamily regulate gene expression programs downstream of the insulin signaling network. It is less clear which proteins mediate transcriptional control exerted by Target of rapamycin (TOR) signaling, but recent studies in nematodes suggest a role for FoxA transcription factors downstream of TOR. In this study we present evidence that outlines a similar connection in Drosophila, in which the FoxA protein Fork head (FKH) regulates cellular and organismal size downstream of TOR. We find that ectopic expression and targeted knockdown of FKH in larval tissues elicits different size phenotypes depending on nutrient state and TOR signaling levels. FKH overexpression has a negative effect on growth under fed conditions, and this phenotype is not further exacerbated by inhibition of TOR via rapamycin feeding. Under conditions of starvation or low TOR signaling levels, knockdown of FKH attenuates the size reduction associated with these conditions. Subcellular localization of endogenous FKH protein is shifted from predominantly cytoplasmic on a high-protein diet to a pronounced nuclear accumulation in animals with reduced levels of TOR or fed with rapamycin. Two putative FKH target genes, CG6770 and cabut, are transcriptionally induced by rapamycin or FKH expression, and silenced by FKH knockdown. Induction of both target genes in heterozygous TOR mutant animals is suppressed by mutations in fkh. Furthermore, TOR signaling levels and FKH impact on transcription of the dFOXO target gene d4E-BP, implying a point of crosstalk with the insulin pathway. In summary, our observations show that an alteration of FKH levels has an effect on cellular and organismal size, and that FKH function is required for the growth inhibition and target gene induction caused by low TOR signaling levels.
Conflict of interest statement
Figures

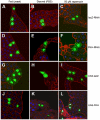


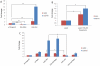
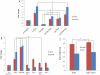
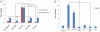

References
-
- Carlsson P, Mahlapuu M. Forkhead transcription factors: key players in development and metabolism. Dev Biol. 2002;250:1–23. - PubMed
-
- Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319–1322. - PubMed
-
- Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, et al. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

