Catabolism of hyaluronan: involvement of transition metals
- PMID: 21217859
- PMCID: PMC2984116
- DOI: 10.2478/v10102-009-0026-y
Catabolism of hyaluronan: involvement of transition metals
Abstract
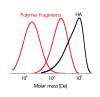
One of the very complex structures in the vertebrates is the joint. The main component of the joint is the synovial fluid with its high-molar-mass glycosaminoglycan hyaluronan, which turnover is approximately twelve hours. Since the synovial fluid does not contain any hyaluronidases, the fast hyaluronan catabolism is caused primarily by reductive-oxidative processes.Eight transition metals - V(23), Mn(25), Fe(26), Co(27), Ni(28), Cu(29), Zn(30), and Mo(42) - naturally occurring in living organism are essential for the control of various metabolic and signaling pathways. They are also the key elements in catabolism of hyaluronan in the joint.In this overview, the role of these metals in physiological and pathophysiological catabolism of hyaluronan is described. The participation of these metals in the initiation and propagation of the radical degradation hyaluronan is critically reviewed.
Keywords: hyaluronan catabolism; joint; oxidative stress; peroxidation; synovial fluid; transition metals.
Figures



References
-
- Buettner GR, Jurkiewicz BA. The ascorbate free radical as a marker of oxidative stress: An EPR study. Free Radic Biol Med. 1993;14:49–55. - PubMed
-
- Delmage JM, Powars DR, Jaynes PK, Allerton SE. The selective suppression of immunogenicity by hyaluronic acid. Annals of Clinical and Laboratory Science. 1986;16:303–310. - PubMed
-
- Feinberg RN, Beebe DC. Hyaluronate in vasculogenesis. Science. 1983;220:1177–1179. - PubMed
-
- Fisher AEO, Naughton DP. Vitamin C contributes to inflammation via radical generating mechanisms: a cautionary note. Medical Hypotheses. 2003;61:657–660. - PubMed
LinkOut - more resources
Full Text Sources
