Solid-state NMR studies of amyloid fibril structure
- PMID: 21219138
- PMCID: PMC3191906
- DOI: 10.1146/annurev-physchem-032210-103539
Solid-state NMR studies of amyloid fibril structure
Abstract
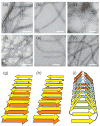
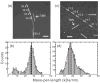
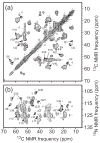
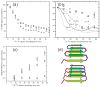
Current interest in amyloid fibrils stems from their involvement in neurodegenerative and other diseases and from their role as an alternative structural state for many peptides and proteins. Solid-state nuclear magnetic resonance (NMR) methods have the unique capability of providing detailed structural constraints for amyloid fibrils, sufficient for the development of full molecular models. In this article, recent progress in the application of solid-state NMR to fibrils associated with Alzheimer's disease, prion fibrils, and related systems is reviewed, along with relevant developments in solid-state NMR techniques and technology.
Figures


References
-
- Sunde M, Blake CCF. From the globular to the fibrous state: Protein structure and structural conversion in amyloid formation. Q Rev Biophys. 1998;31:1–39. - PubMed
-
- Sacchettini JC, Kelly JW. Therapeutic strategies for human amyloid diseases. Nat Rev Drug Discov. 2002;1:267–75. - PubMed
-
- Chiti F, Dobson CM. Amyloid formation by globular proteins under native conditions. Nat Chem Biol. 2009;5:15–22. - PubMed
-
- Tycko R. Molecular structure of amyloid fibrils: Insights from solid state NMR. Q Rev Biophys. 2006;39:1–55. A comprehensive review of solid state NMR studies of amyloid fibrils and relevant methodology up to 2005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

