Immunoglobulin responses at the mucosal interface
- PMID: 21219173
- PMCID: PMC3064559
- DOI: 10.1146/annurev-immunol-031210-101317
Immunoglobulin responses at the mucosal interface
Abstract
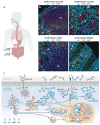
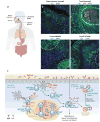
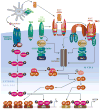
Mucosal surfaces are colonized by large communities of commensal bacteria and represent the primary site of entry for pathogenic agents. To prevent microbial intrusion, mucosal B cells release large amounts of immunoglobulin (Ig) molecules through multiple follicular and extrafollicular pathways. IgA is the most abundant antibody isotype in mucosal secretions and owes its success in frontline immunity to its ability to undergo transcytosis across epithelial cells. In addition to translocating IgA onto the mucosal surface, epithelial cells educate the mucosal immune system as to the composition of the local microbiota and instruct B cells to initiate IgA responses that generate immune protection while preserving immune homeostasis. Here we review recent advances in our understanding of the cellular interactions and signaling pathways governing IgA production at mucosal surfaces and discuss new findings on the regulation and function of mucosal IgD, the most enigmatic isotype of our mucosal antibody repertoire.
Figures
References
-
- Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131–44. - PubMed
-
- Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478–85. - PubMed
-
- Sansonetti PJ. War and peace at mucosal surfaces. Nat Rev Immunol. 2004;4:953–64. - PubMed
-
- Sansonetti PJ. The innate signaling of dangers and the dangers of innate signaling. Nat Immunol. 2006;7:1237–42. - PubMed
-
- Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

