Dysregulation of limbic and auditory networks in tinnitus
- PMID: 21220097
- PMCID: PMC3092532
- DOI: 10.1016/j.neuron.2010.12.002
Dysregulation of limbic and auditory networks in tinnitus
Abstract
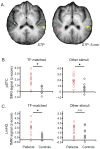
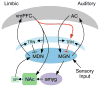
Tinnitus is a common disorder characterized by ringing in the ear in the absence of sound. Converging evidence suggests that tinnitus pathophysiology involves damage to peripheral and/or central auditory pathways. However, whether auditory system dysfunction is sufficient to explain chronic tinnitus is unclear, especially in light of evidence implicating other networks, including the limbic system. Using functional magnetic resonance imaging and voxel-based morphometry, we assessed tinnitus-related functional and anatomical anomalies in auditory and limbic networks. Moderate hyperactivity was present in the primary and posterior auditory cortices of tinnitus patients. However, the nucleus accumbens exhibited the greatest degree of hyperactivity, specifically to sounds frequency-matched to patients' tinnitus. Complementary structural differences were identified in ventromedial prefrontal cortex, another limbic structure heavily connected to the nucleus accumbens. Furthermore, tinnitus-related anomalies were intercorrelated in the two limbic regions and between limbic and primary auditory areas, indicating the importance of auditory-limbic interactions in tinnitus.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



References
-
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. - PubMed
-
- Alain C, Reinke K, McDonald KL, Chau W, Tam F, Pacurar A, Graham S. Left thalamo-cortical network implicated in successful speech separation and identification. Neuroimage. 2005;26:592–599. - PubMed
-
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. - PubMed
-
- Blood AJ, Zatorre RJ, Bermudez P, Evans AC. Emotional responses to pleasant and unpleasant music correlate with activity in paralimbic brain regions. Nat Neurosci. 1999;2:382–387. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

