The dendritic branch is the preferred integrative unit for protein synthesis-dependent LTP
- PMID: 21220104
- PMCID: PMC3032443
- DOI: 10.1016/j.neuron.2010.12.008
The dendritic branch is the preferred integrative unit for protein synthesis-dependent LTP
Abstract
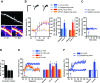
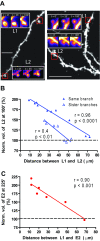
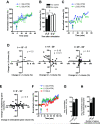
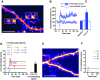
The late-phase of long-term potentiation (L-LTP), the cellular correlate of long-term memory, induced at some synapses facilitates L-LTP expression at other synapses receiving stimulation too weak to induce L-LTP by itself. Using glutamate uncaging and two-photon imaging, we demonstrate that the efficacy of this facilitation decreases with increasing time between stimulations, increasing distance between stimulated spines and with the spines being on different dendritic branches. Paradoxically, stimulated spines compete for L-LTP expression if stimulated too closely together in time. Furthermore, the facilitation is temporally bidirectional but asymmetric. Additionally, L-LTP formation is itself biased toward occurring on spines within a branch. These data support the Clustered Plasticity Hypothesis, which states that such spatial and temporal limits lead to stable engram formation, preferentially at synapses clustered within dendritic branches rather than dispersed throughout the dendritic arbor. Thus, dendritic branches rather than individual synapses are the primary functional units for long-term memory storage.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
LTP-induced long-term stabilization of individual nascent dendritic spines.J Neurosci. 2013 Jan 9;33(2):678-86. doi: 10.1523/JNEUROSCI.1404-12.2013. J Neurosci. 2013. PMID: 23303946 Free PMC article.
-
Locally dynamic synaptic learning rules in pyramidal neuron dendrites.Nature. 2007 Dec 20;450(7173):1195-200. doi: 10.1038/nature06416. Nature. 2007. PMID: 18097401 Free PMC article.
-
Shifting patterns of polyribosome accumulation at synapses over the course of hippocampal long-term potentiation.Hippocampus. 2018 Jun;28(6):416-430. doi: 10.1002/hipo.22841. Epub 2018 Apr 16. Hippocampus. 2018. PMID: 29575288 Free PMC article.
-
BDNF mechanisms in late LTP formation: A synthesis and breakdown.Neuropharmacology. 2014 Jan;76 Pt C:664-76. doi: 10.1016/j.neuropharm.2013.06.024. Epub 2013 Jul 2. Neuropharmacology. 2014. PMID: 23831365 Review.
-
Local protein synthesis, actin dynamics, and LTP consolidation.Curr Opin Neurobiol. 2008 Oct;18(5):524-31. doi: 10.1016/j.conb.2008.09.013. Epub 2008 Oct 14. Curr Opin Neurobiol. 2008. PMID: 18834940 Review.
Cited by
-
Glutamate-induced RNA localization and translation in neurons.Proc Natl Acad Sci U S A. 2016 Nov 1;113(44):E6877-E6886. doi: 10.1073/pnas.1614267113. Epub 2016 Oct 17. Proc Natl Acad Sci U S A. 2016. PMID: 27791158 Free PMC article.
-
An interactive time series image analysis software for dendritic spines.Sci Rep. 2022 Jul 20;12(1):12405. doi: 10.1038/s41598-022-16137-y. Sci Rep. 2022. PMID: 35859092 Free PMC article.
-
Tag and capture: how salient experiences target and rescue nearby events in memory.Trends Cogn Sci. 2022 Sep;26(9):782-795. doi: 10.1016/j.tics.2022.06.009. Epub 2022 Jul 13. Trends Cogn Sci. 2022. PMID: 35842373 Free PMC article. Review.
-
BitBrain and Sparse Binary Coincidence (SBC) memories: Fast, robust learning and inference for neuromorphic architectures.Front Neuroinform. 2023 Mar 21;17:1125844. doi: 10.3389/fninf.2023.1125844. eCollection 2023. Front Neuroinform. 2023. PMID: 37025552 Free PMC article.
-
The temporal pattern of synaptic activation determines the longevity of structural plasticity at dendritic spines.iScience. 2023 May 8;26(6):106835. doi: 10.1016/j.isci.2023.106835. eCollection 2023 Jun 16. iScience. 2023. PMID: 37332599 Free PMC article.
References
-
- Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. - PubMed
-
- Chutkow JG. Metabolism of magnesium in central nervous system. Relationship between concentrations of magnesium in cerebrospinal fluid and brain in magnesium deficiency. Neurology. 1974;24:780–787. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

