Phosphorylation of glutamyl-prolyl tRNA synthetase by cyclin-dependent kinase 5 dictates transcript-selective translational control
- PMID: 21220307
- PMCID: PMC3029695
- DOI: 10.1073/pnas.1011275108
Phosphorylation of glutamyl-prolyl tRNA synthetase by cyclin-dependent kinase 5 dictates transcript-selective translational control
Abstract
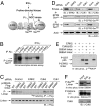
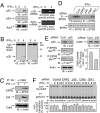
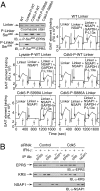
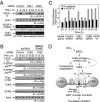
Cyclin-dependent kinase 5 (Cdk5) is an atypical but essential member of the Cdk kinase family, and its dysregulation or deletion has been implicated in inflammation-related disorders by an undefined mechanism. Here we show that Cdk5 is an indispensable activator of the GAIT (IFN-γ-activated inhibitor of translation) pathway, which suppresses expression of a posttranscriptional regulon of proinflammatory genes in myeloid cells. Through induction of its regulatory protein, Cdk5R1 (p35), IFN-γ activates Cdk5 to phosphorylate Ser(886) in the linker domain of glutamyl-prolyl tRNA synthetase (EPRS), the initial event in assembly of the GAIT complex. Cdk5/p35 also induces, albeit indirectly via a distinct kinase, phosphorylation of Ser(999), the second essential event in GAIT pathway activation. Diphosphorylated EPRS is released from its residence in the tRNA multisynthetase complex for immediate binding to NS1-associated protein and subsequent binding to ribosomal protein L13a and GAPDH. The mature heterotetrameric GAIT complex binds the 3' UTR GAIT element of VEGF-A and other target mRNAs and suppresses their translation in myeloid cells. Inhibition of Cdk5/p35 inhibits both EPRS phosphorylation events, prevents EPRS release from the tRNA multisynthetase complex, and blocks translational suppression of GAIT element-bearing mRNAs, resulting in increased expression of inflammatory proteins. Our study reveals a unique role of Cdk5/p35 in activation of the major noncanonical function of EPRS, namely translational control of macrophage inflammatory gene expression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ibba M, Söll D. Aminoacyl-tRNA synthesis. Annu Rev Biochem. 2000;69:617–650. - PubMed
-
- Ribas de Pouplana L, Schimmel P. Aminoacyl-tRNA synthetases: Potential markers of genetic code development. Trends Biochem Sci. 2001;26:591–596. - PubMed
-
- Lee SW, Cho BH, Park SG, Kim S. Aminoacyl-tRNA synthetase complexes: Beyond translation. J Cell Sci. 2004;117:3725–3734. - PubMed
-
- Clemens MJ. Does protein phosphorylation play a role in translational control by eukaryotic aminoacyl-tRNA synthetases? Trends Biochem Sci. 1990;15:172–175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

