Deep bradycardia and heart block caused by inducible cardiac-specific knockout of the pacemaker channel gene Hcn4
- PMID: 21220308
- PMCID: PMC3029742
- DOI: 10.1073/pnas.1010122108
Deep bradycardia and heart block caused by inducible cardiac-specific knockout of the pacemaker channel gene Hcn4
Abstract
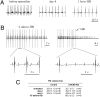
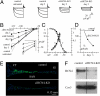
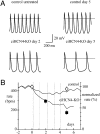
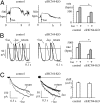
Cardiac pacemaking generation and modulation rely on the coordinated activity of several processes. Although a wealth of evidence indicates a relevant role of the I(f) ("funny," or pacemaker) current, whose molecular constituents are the hyperpolarization-activated, cyclic nucleotide-gated (HCN) channels and particularly HCN4, work with mice where Hcn genes were knocked out, or functionally modified, has challenged this view. However, no previous studies used a cardiac-specific promoter to induce HCN4 ablation in adult mice. We report here that, in an inducible and cardiac-specific HCN4 knockout (ciHCN4-KO) mouse model, ablation of HCN4 consistently leads to progressive development of severe bradycardia (∼50% reduction of original rate) and AV block, eventually leading to heart arrest and death in about 5 d. In vitro analysis of sinoatrial node (SAN) myocytes isolated from ciHCN4-KO mice at the mean time of death revealed a strong reduction of both the I(f) current (by ∼70%) and of the spontaneous rate (by ∼60%). In agreement with functional results, immunofluorescence and Western blot analysis showed reduced expression of HCN4 protein in SAN tissue and cells. In ciHCN4-KO animals, the residual I(f) was normally sensitive to β-adrenergic receptor (β-AR) modulation, and the permanence of rate response to β-AR stimulation was observed both in vivo and in vitro. Our data show that cardiac HCN4 channels are essential for normal heart impulse generation and conduction in adult mice and support the notion that dysfunctional HCN4 channels can be a direct cause of rhythm disorders. This work contributes to identifying the molecular mechanism responsible for cardiac pacemaking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Mangoni ME, Nargeot J. Genesis and regulation of the heart automaticity. Physiol Rev. 2008;88:919–982. - PubMed
-
- Brioschi C, et al. Distribution of the pacemaker HCN4 channel mRNA and protein in the rabbit sinoatrial node. J Mol Cell Cardiol. 2009;47:221–227. - PubMed
-
- Tellez JO, et al. Differential expression of ion channel transcripts in atrial muscle and sinoatrial node in rabbit. Circ Res. 2006;99:1384–1393. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

