Density functional theory in surface chemistry and catalysis
- PMID: 21220337
- PMCID: PMC3024687
- DOI: 10.1073/pnas.1006652108
Density functional theory in surface chemistry and catalysis
Abstract
Recent advances in the understanding of reactivity trends for chemistry at transition-metal surfaces have enabled in silico design of heterogeneous catalysts in a few cases. The current status of the field is discussed with an emphasis on the role of coupling theory and experiment and future challenges.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
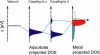

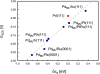
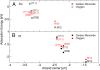

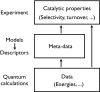

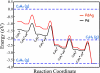
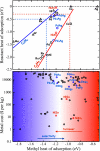
References
-
- Maxwell IE. Driving forces for studies in catalysis. Stud Surf Sci Catal. 1996;101:1–9.
-
- Office of Basic Energy Sciences, US Department of Energy. Basic Energy Needs for Solar Energy Utilization, Workshop Report. 2005. Available at http://www.er.doe.gov/bes/reports. Accessed June 1, 2010.
-
- Office of Basic Energy Sciences, US Department of Energy. Basic Energy Needs Catalysis for Energy, Workshop Report. 2007. Available at http://www.er.doe.gov/bes/reports. Accessed June 1, 2010.
-
- Valden M, Lai X, Goodman DW. Onset of catalytic activity of gold clusters on titania with the appearance of nonmetallic properties. Science. 1998;281:1647–1650. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

