Translocation of surface-localized effectors in type III secretion
- PMID: 21220342
- PMCID: PMC3029700
- DOI: 10.1073/pnas.1013888108
Translocation of surface-localized effectors in type III secretion
Abstract
Pathogenic Yersinia species suppress the host immune response by using a plasmid-encoded type III secretion system (T3SS) to translocate virulence proteins into the cytosol of the target cells. T3SS-dependent protein translocation is believed to occur in one step from the bacterial cytosol to the target-cell cytoplasm through a conduit created by the T3SS upon target cell contact. Here, we report that T3SS substrates on the surface of Yersinia pseudotuberculosis are translocated into target cells. Upon host cell contact, purified YopH coated on Y. pseudotuberculosis was specifically and rapidly translocated across the target-cell membrane, which led to a physiological response in the infected cell. In addition, translocation of externally added YopH required a functional T3SS and a specific translocation domain in the effector protein. Efficient, T3SS-dependent translocation of purified YopH added in vitro was also observed when using coated Salmonella typhimurium strains, which implies that T3SS-mediated translocation of extracellular effector proteins is conserved among T3SS-dependent pathogens. Our results demonstrate that polarized T3SS-dependent translocation of proteins can be achieved through an intermediate extracellular step that can be reconstituted in vitro. These results indicate that translocation can occur by a different mechanism from the assumed single-step conduit model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

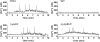

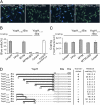
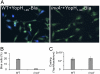
Comment in
-
Translocation of effectors: revisiting the injectosome model.Future Microbiol. 2011 May;6(5):483-4. doi: 10.2217/fmb.11.33. Future Microbiol. 2011. PMID: 21585256 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

