Recombinant erythroid Kruppel-like factor fused to GATA1 up-regulates delta- and gamma-globin expression in erythroid cells
- PMID: 21220744
- PMCID: PMC3062308
- DOI: 10.1182/blood-2010-07-294751
Recombinant erythroid Kruppel-like factor fused to GATA1 up-regulates delta- and gamma-globin expression in erythroid cells
Abstract
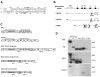
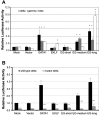
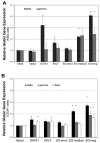
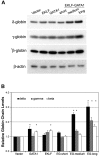
The β-hemoglobinopathies sickle cell disease and β-thalassemia are among the most common human genetic disorders worldwide. Hemoglobin A2 (HbA2, α₂δ₂) and fetal hemoglobin (HbF, α₂γ₂) both inhibit the polymerization of hemoglobin S, which results in erythrocyte sickling. Expression of erythroid Kruppel-like factor (EKLF) and GATA1 is critical for transitioning hemoglobin from HbF to hemoglobin A (HbA, α₂β₂) and HbA2. The lower levels of δ-globin expression compared with β-globin expression seen in adulthood are likely due to the absence of an EKLF-binding motif in the δ-globin proximal promoter. In an effort to up-regulate δ-globin to increase HbA2 expression, we created a series of EKLF-GATA1 fusion constructs composed of the transactivation domain of EKLF and the DNA-binding domain of GATA1, and then tested their effects on hemoglobin expression. EKLF-GATA1 fusion proteins activated δ-, γ-, and β-globin promoters in K562 cells, and significantly up-regulated δ- and γ-globin RNA transcript and protein expression in K562 and/or CD34(+) cells. The binding of EKLF-GATA1 fusion proteins at the GATA1 consensus site in the δ-globin promoter was confirmed by chromatin immunoprecipitation assay. Our studies demonstrate that EKLF-GATA1 fusion proteins can enhance δ-globin expression through interaction with the δ-globin promoter, and may represent a new genetic therapeutic approach to β-hemoglobinopathies.
Figures

References
-
- Platt OS. Hydroxyurea for the treatment of sickle cell anemia. N Engl J Med. 2008;358(13):1362–1369. - PubMed
-
- Hsieh MM, Tisdale TJ, Rodgers GP. Hemolytic anemia: thalassemias and sickle cell disease. In: Rodgers GP, Young NS, editors. Bethesda Handbook of Clinical Hematology. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009. pp. 35–54.
-
- Rodgers GP, Saunthararajah Y. Advances in experimental treatment of beta-thalassaemia. Expert Opin Investig Drugs. 2001;10(5):925–934. - PubMed
-
- Rodgers GP, Dover GJ, Noguchi CT, Schechter AN, Nienhuis AW. Hematologic responses of patients with sickle cell disease to treatment with hydroxyurea. N Engl J Med. 1990;322(15):1037–1045. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

