U.S. multicenter pilot study of daily consensus interferon (CIFN) plus ribavirin for "difficult-to-treat" HCV genotype 1 patients
- PMID: 21221804
- PMCID: PMC3041922
- DOI: 10.1007/s10620-010-1504-y
U.S. multicenter pilot study of daily consensus interferon (CIFN) plus ribavirin for "difficult-to-treat" HCV genotype 1 patients
Abstract
Background: Patients with chronic hepatitis C genotype 1 (HCV-1) and difficult-to-treat characteristics respond poorly to pegylated interferon alfa and ribavirin (RBV), and could benefit from an interferon with increased activity (consensus interferon or CIFN), favorable viral kinetics from daily dosing, and a longer duration of therapy. The purpose of this pilot study was to determine the efficacy and safety of daily CIFN + RBV for initial treatment of patients with HCV-1 infection.
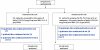
Methods: Patients with difficult-to-treat characteristics (92% male, 33% African American, 78% Veterans Affairs [VA]; 67% high viral load, 59% stage 3-4 fibrosis, and mean weight of 204 lbs) were enrolled at seven VA and two community medical centers. They were randomized to daily CIFN (15 mcg/day SQ) and RBV (1-1.2 g/d PO) given for either 52 weeks (group A, n = 33) or 52-72 weeks (from time of viral response +48 weeks) (group B, n = 31).
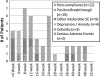
Results: Intention to treat analysis for treatment groups A and B demonstrated 33% (11/33) and 32% (10/31) sustained virologic response (SVR), respectively. Only 2/31 patients in group B received more than 52 weeks of treatment. The overall group demonstrated a 31% (20/64) rapid virologic response rate (RVR), 54% (34/64) end of treatment virologic response and a 33% (21/64) SVR. Patients with RVR at 4 weeks, early virologic response from 8-12 weeks, and late virologic response from 16-24 weeks demonstrated SVR of 75% (15/20), 31% (4/13), and 22% (2/9), respectively. Overall early non-protocol discontinuation occurred in 26/64 (40%) patients.
Conclusion: Daily CIFN and ribavirin for initial treatment of HCV-1 patients has potential for achieving a relatively high RVR rate, but discontinuations are frequent and successful use of this regimen is highly dependent on adequate patient support to maintain adherence.
Figures



Comment in
-
Consensus interferon: tailored therapy and the impact of adherence.Dig Dis Sci. 2011 Mar;56(3):631-4. doi: 10.1007/s10620-011-1563-8. Dig Dis Sci. 2011. PMID: 21259073 No abstract available.
Similar articles
-
Retreatment of hepatitis C with consensus interferon and ribavirin after nonresponse or relapse to pegylated interferon and ribavirin: a national VA clinical practice study.Dig Dis Sci. 2011 Aug;56(8):2439-48. doi: 10.1007/s10620-011-1746-3. Epub 2011 Jun 2. Dig Dis Sci. 2011. PMID: 21633833
-
Cost-efficacy analysis of peginterferon alfa-2b plus ribavirin compared with peginterferon alfa-2a plus ribavirin for the treatment of chronic hepatitis C.J Manag Care Pharm. 2005 Oct;11(8):687-94. doi: 10.18553/jmcp.2005.11.8.687. J Manag Care Pharm. 2005. PMID: 16194133 Free PMC article.
-
The safety and tolerability of daily infergen plus ribavirin in the treatment of naíïve chronic hepatitis C patients.J Viral Hepat. 2003 Jan;10(1):55-60. doi: 10.1046/j.1365-2893.2003.00402.x. J Viral Hepat. 2003. PMID: 12558913 Clinical Trial.
-
Treatment with daily consensus interferon (CIFN) plus ribavirin in non-responder patients with chronic hepatitis C: a randomized open-label pilot study.J Hepatol. 2006 Feb;44(2):291-301. doi: 10.1016/j.jhep.2005.10.021. Epub 2005 Nov 28. J Hepatol. 2006. PMID: 16360972 Clinical Trial.
-
Simeprevir increases rate of sustained virologic response among treatment-experienced patients with HCV genotype-1 infection: a phase IIb trial.Gastroenterology. 2014 Feb;146(2):430-41.e6. doi: 10.1053/j.gastro.2013.10.058. Epub 2013 Nov 1. Gastroenterology. 2014. PMID: 24184810 Clinical Trial.
Cited by
-
Consensus interferon: tailored therapy and the impact of adherence.Dig Dis Sci. 2011 Mar;56(3):631-4. doi: 10.1007/s10620-011-1563-8. Dig Dis Sci. 2011. PMID: 21259073 No abstract available.
-
Differences in clinical outcomes among hepatitis C genotype 1-infected patients treated with peginterferon alpha-2a or peginterferon alpha-2b plus ribavirin: a meta-analysis.Clin Exp Gastroenterol. 2012;5:11-21. doi: 10.2147/CEG.S28253. Epub 2012 Feb 14. Clin Exp Gastroenterol. 2012. PMID: 22427726 Free PMC article.
-
Molecular epidemiology of hepatitis C virus and its relation with persistence or clearance of infection in Hamadan, West-Iran.Iran J Microbiol. 2015 Apr;7(2):109-17. Iran J Microbiol. 2015. PMID: 26622972 Free PMC article.
-
What is the future of ribavirin therapy for hepatitis C?Antiviral Res. 2014 Apr;104:34-9. doi: 10.1016/j.antiviral.2014.01.005. Epub 2014 Jan 25. Antiviral Res. 2014. PMID: 24468277 Free PMC article. Review.
References
-
- Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. - PubMed
-
- NIH National Institutes of Health consensus development conference statement: management of hepatitis C: 2002. Hepatology. 2002;36:S3–S15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

