Complete genome sequencing and analysis of a Lancefield group G Streptococcus dysgalactiae subsp. equisimilis strain causing streptococcal toxic shock syndrome (STSS)
- PMID: 21223537
- PMCID: PMC3027156
- DOI: 10.1186/1471-2164-12-17
Complete genome sequencing and analysis of a Lancefield group G Streptococcus dysgalactiae subsp. equisimilis strain causing streptococcal toxic shock syndrome (STSS)
Abstract
Background: Streptococcus dysgalactiae subsp. equisimilis (SDSE) causes invasive streptococcal infections, including streptococcal toxic shock syndrome (STSS), as does Lancefield group A Streptococcus pyogenes (GAS). We sequenced the entire genome of SDSE strain GGS_124 isolated from a patient with STSS.
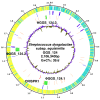
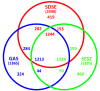
Results: We found that GGS_124 consisted of a circular genome of 2,106,340 bp. Comparative analyses among bacterial genomes indicated that GGS_124 was most closely related to GAS. GGS_124 and GAS, but not other streptococci, shared a number of virulence factor genes, including genes encoding streptolysin O, NADase, and streptokinase A, distantly related to SIC (DRS), suggesting the importance of these factors in the development of invasive disease. GGS_124 contained 3 prophages, with one containing a virulence factor gene for streptodornase. All 3 prophages were significantly similar to GAS prophages that carry virulence factor genes, indicating that these prophages had transferred these genes between pathogens. SDSE was found to contain a gene encoding a superantigen, streptococcal exotoxin type G, but lacked several genes present in GAS that encode virulence factors, such as other superantigens, cysteine protease speB, and hyaluronan synthase operon hasABC. Similar to GGS_124, the SDSE strains contained larger numbers of clustered, regularly interspaced, short palindromic repeats (CRISPR) spacers than did GAS, suggesting that horizontal gene transfer via streptococcal phages between SDSE and GAS is somewhat restricted, although they share phage species.
Conclusion: Genome wide comparisons of SDSE with GAS indicate that SDSE is closely and quantitatively related to GAS. SDSE, however, lacks several virulence factors of GAS, including superantigens, SPE-B and the hasABC operon. CRISPR spacers may limit the horizontal transfer of phage encoded GAS virulence genes into SDSE. These findings may provide clues for dissecting the pathological roles of the virulence factors in SDSE and GAS that cause STSS.
Figures





References
-
- Bramhachari PV, Kaul SY, McMillan DJ, Shaila MS, Karmarkar MG, Sriprakash KS. Disease burden due to Streptococcus dysgalactiae subsp. equisimilis (group G and C streptococcus) is higher than that due to Streptococcus pyogenes among Mumbai school children. J Med Microbiol. 2010;59:220–223. doi: 10.1099/jmm.0.015644-0. - DOI - PubMed
-
- Lopardo HA, Vidal P, Sparo M, Jeric P, Centron D, Facklam RR, Paganini H, Pagniez NG, Lovgren M, Beall B. Six-month multicenter study on invasive infections due to Streptococcus pyogenes and Streptococcus dysgalactiae subsp. equisimilis in Argentina. J Clin Microbiol. 2005;43:802–807. doi: 10.1128/JCM.43.2.802-807.2005. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

