Toll-like receptor 3 upregulation by type I interferon in healthy and scleroderma dermal fibroblasts
- PMID: 21223583
- PMCID: PMC3241348
- DOI: 10.1186/ar3221
Toll-like receptor 3 upregulation by type I interferon in healthy and scleroderma dermal fibroblasts
Abstract
Introduction: Increased levels of genes in the type I interferon (IFN) pathway have been observed in patients with systemic sclerosis (SSc), or scleroderma. How type I IFN regulates the dermal fibroblast and its participation in the development of dermal fibrosis is not known. We hypothesized that one mechanism by which type I IFN may contribute to dermal fibrosis is through upregulation of specific Toll-like receptors (TLRs) on dermal fibroblasts. Therefore, we investigated the regulation of TLR expression on dermal fibroblasts by IFN.
Methods: The expression of TLRs was assessed in cultured dermal fibroblasts from control and SSc patients stimulated with IFNα2. The ability of IFNα2 to regulate TLR-induced interleukin (IL)-6 and CC chemokine ligand 2 production was also assessed. Immunohistochemical analyses were performed to determine whether TLR3 was expressed in skin biopsies in the bleomycin-induced skin fibrosis model and in patients with SSc.
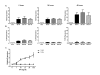
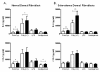
Results: IFNα2 increased TLR3 expression on human dermal fibroblasts, which resulted in enhanced TLR3-induced IL-6 production. SSc fibroblasts have an augmented TLR3 response to IFNα2 relative to control fibroblasts. Pretreatment of fibroblasts with transforming growth factor (TGF)-β increased TLR3 induction by IFNα2, but coincubation of TGF-β did not alter TLR3 induction by IFN. Furthermore, IFNα2 inhibits but does not completely block the induction of connective tissue growth factor and collagen expression by TGF-βin fibroblasts. TLR3 expression was observed in dermal fibroblasts and inflammatory cells from skin biopsies from patients with SSc as well as in the bleomycin-induced skin fibrosis model.
Conclusions: Type I IFNs can increase the inflammatory potential of dermal fibroblasts through the upregulation of TLR3.
Figures



References
-
- Varga J. Systemic sclerosis: an update. Bull NYU Hosp Jt Dis. 2008;66:198–202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

