Cytoglobin, a novel member of the globin family, protects kidney fibroblasts against oxidative stress under ischemic conditions
- PMID: 21224051
- PMCID: PMC3069879
- DOI: 10.1016/j.ajpath.2010.11.011
Cytoglobin, a novel member of the globin family, protects kidney fibroblasts against oxidative stress under ischemic conditions
Abstract
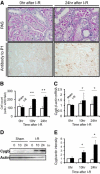
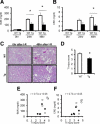
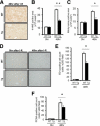
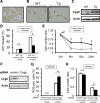
Cytoglobin (Cygb) is a novel member of the vertebrate globin superfamily. Although it is expressed in splanchnic fibroblasts of various organs, details of its function remain unknown. In the present study, kidney ischemia-reperfusion (I/R) increased the number of Cygb-positive cells per area and up-regulated Cygb mRNA and protein expression in kidney cortex tissues. Similarly, hypoxia up-regulated Cygb expression in cultured rat kidney fibroblasts. The biological function of Cygb in vivo was evaluated in Cygb-overexpressing transgenic rats. Renal dysfunction and histologic damage after renal I/R were ameliorated (mean [SE] serum urea nitrogen concentration after I/R injury, 260.6 [44.9] mg/dL in wild-type rats versus 101.0 [36.0] mg/dL in transgenic rats; P < 0.05) in association with improvement of oxidative stress. Primary cultured fibroblasts from Cygb transgenic rat kidney were resistant to exogenous oxidant stimuli, and treatment of immortalized kidney fibroblasts with Cygb-small interfering RNA (siRNA) enhanced cellular oxidant stress and subsequently decreased cell viability (cell count ratio after exposure to hydrogen peroxide, 35.9% [1.6%] in control-siRNA-treated cells versus 25.5% [2.0%] in Cygb-siRNA-treated cells; P < 0.05). Further, chemical or mutant disruption of heme in Cygb impaired its antioxidant properties, which suggests that the heme of Cygb per se possesses a radical scavenging function. These findings show for the first time, to our knowledge, that Cygb serves as a defensive mechanism against oxidative stress both in vitro and in vivo.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Wittenberg B.A., Wittenberg J.B. Transport of oxygen in muscle. Annu Rev Physiol. 1989;51:857–878. - PubMed
-
- Kawada N., Kristensen D.B., Asahina K., Nakatani K., Minamiyama Y., Seki S., Yoshizato K. Characterization of a stellate cell activation-associated protein (STAP) with peroxidase activity found in rat hepatic stellate cells. J Biol Chem. 2001;276:25318–25323. - PubMed
-
- Trent J.T., III, Hargrove M.S. A ubiquitously expressed human hexacoordinate hemoglobin. J Biol Chem. 2002;277:19538–19545. - PubMed
-
- Burmester T., Ebner B., Weich B., Hankeln T. Cytoglobin: a novel globin type ubiquitously expressed in vertebrate tissues. Mol Biol Evol. 2002;19:416–421. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

