Tissue eosinophilia in a mouse model of colitis is highly dependent on TLR2 and independent of mast cells
- PMID: 21224053
- PMCID: PMC3069897
- DOI: 10.1016/j.ajpath.2010.11.041
Tissue eosinophilia in a mouse model of colitis is highly dependent on TLR2 and independent of mast cells
Abstract
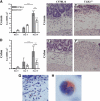
The mechanisms initiating eosinophil influx into sites of inflammation have been well studied in allergic disease but are poorly understood in other settings. This study examined the roles of TLR2 and mast cells in eosinophil accumulation during a nonallergic model of eosinophilia-associated colitis. TLR2-deficient mice (TLR2(-/-)) developed a more severe colitis than wild-type mice in the dextran sodium sulfate (DSS) model. However, they had significantly fewer eosinophils in the submucosa of the cecum (P < 0.01) and mid-colon (P < 0.01) than did wild-type mice after DSS treatment. Decreased eosinophilia in TLR2(-/-) mice was associated with lower levels of cecal CCL11 (P < 0.01). Peritoneal eosinophils did not express TLR2 protein, but TLR2 ligand injection into the peritoneal cavity induced local eosinophil recruitment, indicating that TLR2 activation of other cell types can mediate eosinophil recruitment. After DSS treatment, mast cell-deficient (Kit(W-sh/W-sh)) mice had similar levels of intestinal tissue eosinophilia were observed as those in wild-type mice. However, mast cell-deficient mice were partially protected from DSS-induced weight loss, an effect that was reversed by mast cell reconstitution. Overall, this study indicates a critical role for indirect TLR2-dependent pathways, but not mast cells, in the generation of eosinophilia in the large intestine during experimental colitis and has important implications for the regulation of eosinophils at mucosal inflammatory sites.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Xavier R.J., Podolsky D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. - PubMed
-
- Rothenberg M.E., Mishra A., Brandt E.B., Hogan S.P. Gastrointestinal eosinophils. Immunol Rev. 2001;179:139–155. - PubMed
-
- King T., Biddle W., Bhatia P., Moore J., Miner P.B., Jr Colonic mucosal mast cell distribution at line of demarcation of active ulcerative colitis. Dig Dis Sci. 1992;37:490–495. - PubMed
-
- Nishida Y., Murase K., Isomoto H., Furusu H., Mizuta Y., Riddell R.H., Kohno S. Different distribution of mast cells and macrophages in colonic mucosa of patients with collagenous colitis and inflammatory bowel disease. Hepatogastroenterology. 2002;49:678–682. - PubMed
-
- Weller P.F. Eosinophils: structure and functions. Curr Opin Immunol. 1994;6:85–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

