Bile acids induce inflammatory genes in hepatocytes: a novel mechanism of inflammation during obstructive cholestasis
- PMID: 21224055
- PMCID: PMC3070591
- DOI: 10.1016/j.ajpath.2010.11.026
Bile acids induce inflammatory genes in hepatocytes: a novel mechanism of inflammation during obstructive cholestasis
Abstract
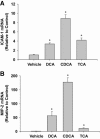
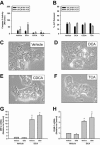
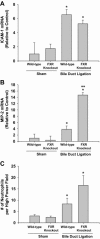
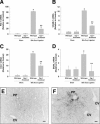
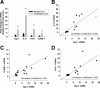
Inflammation contributes to liver injury during cholestasis. The mechanism by which cholestasis initiates an inflammatory response in the liver, however, is not known. Two hypotheses were investigated in the present studies. First, activation of Toll-like receptor 4 (TLR4), either by bacterial lipopolysaccharide or by damage-associated molecular pattern molecules released from dead hepatocytes, triggers an inflammatory response. Second, bile acids act as inflammagens, and directly activate signaling pathways in hepatocytes that stimulate production of proinflammatory mediators. Liver inflammation was not affected in lipopolysaccharide-resistant C3H/HeJ mice after bile duct ligation, indicating that Toll-like receptor 4 is not required for initiation of inflammation. Treatment of hepatocytes with bile acids did not directly cause cell toxicity but increased the expression of numerous proinflammatory mediators, including cytokines, chemokines, adhesion molecules, and other proteins that influence immune cell levels and function. Up-regulation of several of these genes in hepatocytes and in the liver after bile duct ligation required early growth response factor-1, but not farnesoid X receptor. In addition, early growth response factor-1 was up-regulated in the livers of patients with cholestasis and correlated with levels of inflammatory mediators. These data demonstrate that Toll-like receptor 4 is not required for the initiation of acute inflammation during cholestasis. In contrast, bile acids directly activate a signaling network in hepatocytes that promotes hepatic inflammation during cholestasis.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures

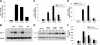

References
-
- Li M.K., Crawford J.M. The pathology of cholestasis. Semin Liver Dis. 2004;24:21–42. - PubMed
-
- Qureshi W.A. Intrahepatic cholestatic syndromes: pathogenesis, clinical features and management. Dig Dis. 1999;17:49–59. - PubMed
-
- Setchell K.D., Rodrigues C.M., Clerici C., Solinas A., Morelli A., Gartung C., Boyer J. Bile acid concentrations in human and rat liver tissue and in hepatocyte nuclei. Gastroenterology. 1997;112:226–235. - PubMed
-
- Lindblad L., Lundholm K., Schersten T. Bile acid concentrations in systemic and portal serum in presumably normal man and in cholestatic and cirrhotic conditions. Scand J Gastroenterol. 1977;12:395–400. - PubMed
-
- Gujral J.S., Farhood A., Bajt M.L., Jaeschke H. Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology. 2003;38:355–363. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

