Astrocytes control GABAergic inhibition of neurons in the mouse barrel cortex
- PMID: 21224221
- PMCID: PMC3060594
- DOI: 10.1113/jphysiol.2010.203224
Astrocytes control GABAergic inhibition of neurons in the mouse barrel cortex
Abstract
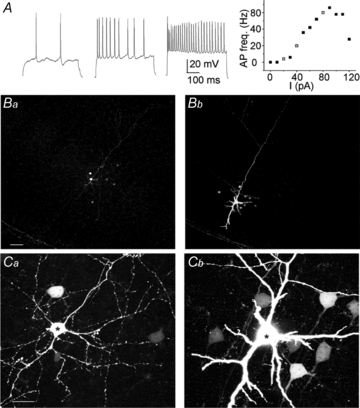
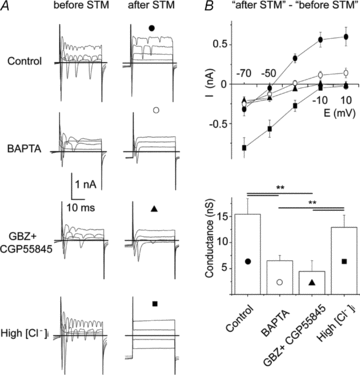
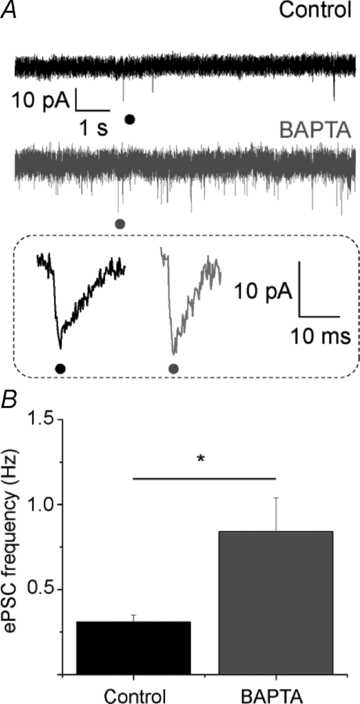
Astrocytes in the barrel cortex respond with a transient Ca2+ increase to neuronal stimulation and this response is restricted to the stimulated barrel field. In the present study we suppressed the astrocyte response by dialysing these cells with the Ca2+ chelator BAPTA. Electrical stimulation triggered a depolarization in stellate or pyramidal ‘regular spiking' neurons from cortex layer 4 and 2/3 and this response was augmented in amplitude and duration after astrocytes were dialysed with BAPTA. Combined blockade of GABAA and GABAB receptors mimicked the effect of BAPTA dialysis, while glutamate receptor blockers had no effect. Moreover, the frequency of spontaneous postsynaptic currents was increased after BAPTA dialysis. Outside the range of BAPTA dialysis astrocytes responded with a Ca2+ increase, but in contrast to control, the response was no longer restricted to one barrel field. Our findings indicate that astrocytes control neuronal inhibition in the barrel cortex.
Figures




References
-
- Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience. 1991;41:365–379. - PubMed
-
- Agulhon C, Fiacco TA, McCarthy KD. Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science. 2010;327:1250–1254. - PubMed
-
- Bacci A, Sancini G, Verderio C, Armano S, Pravettoni E, Fesce R, Franceschetti S, Matteoli M. Block of glutamate-glutamine cycle between astrocytes and neurons inhibits epileptiform activity in hippocampus. J Neurophysiol. 2002;88:2302–2310. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

