Hippo pathway-independent restriction of TAZ and YAP by angiomotin
- PMID: 21224387
- PMCID: PMC3044958
- DOI: 10.1074/jbc.C110.212621
Hippo pathway-independent restriction of TAZ and YAP by angiomotin
Abstract
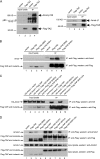
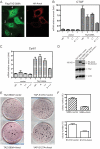
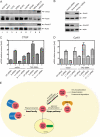
The Hippo pathway restricts the activity of transcriptional co-activators TAZ and YAP by phosphorylating them for cytoplasmic sequestration or degradation. In this report, we describe an independent mechanism for the cell to restrict the activity of TAZ and YAP through interaction with angiomotin (Amot) and angiomotin-like 1 (AmotL1). Amot and AmotL1 were robustly co-immunoprecipitated with FLAG-tagged TAZ, and their interaction is dependent on the WW domain of TAZ and the PPXY motif in the N terminus of Amot. Amot and AmotL1 also interact with YAP via the first WW domain of YAP. Overexpression of Amot and AmotL1 caused cytoplasmic retention of TAZ and suppressed its transcriptional outcome such as the expression of CTGF and Cyr61. Hippo refractory TAZ mutant (S89A) is also negatively regulated by Amot and AmotL1. HEK293 cells express the highest level of Amot and AmotL1 among nine cell lines examined, and silencing the expression of endogenous Amot increased the expression of CTGF and Cyr61 either at basal levels or upon overexpression of exogenous S89A. These results reveal a novel mechanism to restrict the activity of TAZ and YAP through physical interaction with Amot and AmotL1.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

