Integrin α5β1 facilitates cancer cell invasion through enhanced contractile forces
- PMID: 21224397
- PMCID: PMC3021998
- DOI: 10.1242/jcs.071985
Integrin α5β1 facilitates cancer cell invasion through enhanced contractile forces
Abstract
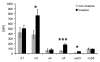
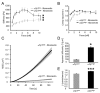
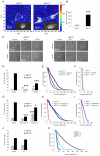
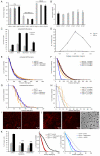
Cell migration through connective tissue, or cell invasion, is a fundamental biomechanical process during metastasis formation. Cell invasion usually requires cell adhesion to the extracellular matrix through integrins. In some tumors, increased integrin expression is associated with increased malignancy and metastasis formation. Here, we have studied the invasion of cancer cells with different α5β1 integrin expression levels into loose and dense 3D collagen fiber matrices. Using a cell sorter, we isolated from parental MDA-MB-231 breast cancer cells two subcell lines expressing either high or low amounts of α5β1 integrins (α5β1(high) or α5β1(low) cells, respectively). α5β1(high) cells showed threefold increased cell invasiveness compared to α5β1(low) cells. Similar results were obtained for 786-O kidney and T24 bladder carcinoma cells, and cells in which the α5 integrin subunit was knocked down using specific siRNA. Knockdown of the collagen receptor integrin subunit α2 also reduced invasiveness, but to a lesser degree than knockdown of integrin subunit α5. Fourier transform traction microscopy revealed that the α5β1(high) cells generated sevenfold greater contractile forces than α5β1(low) cells. Cell invasiveness was reduced after addition of the myosin light chain kinase inhibitor ML-7 in α5β1(high) cells, but not in α5β1(low) cells, suggesting that α5β1 integrins enhance cell invasion through enhanced transmission and generation of contractile forces.
Figures





References
-
- Al-Mehdi A. B., Tozawa K., Fisher A. B., Shientag L., Lee A., Muschel R. J. (2000). Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nat. Med. 6, 100-102 - PubMed
-
- Balaban N. Q., Schwarz U. S., Riveline D., Goichberg P., Tzur G., Sabanay I., Mahalu D., Safran S., Bershadsky A., Addadi L., et al. (2001). Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 3, 466-472 - PubMed
-
- Batlle E., Sancho E., Franci C., Dominguez D., Monfar M., Baulida J., Garcia De Herreros A. (2000). The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell Biol. 2, 84-89 - PubMed
-
- Bauer K., Mierke C., Behrens J. (2007). Expression profiling reveals genes associated with transendothelial migration of tumor cells: a functional role for alpha v beta3 integrin. Int. J. Cancer 121, 1910-1918 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

