Importance of single molecular determinants in the fidelity of expanded genetic codes
- PMID: 21224416
- PMCID: PMC3029751
- DOI: 10.1073/pnas.1012276108
Importance of single molecular determinants in the fidelity of expanded genetic codes
Abstract
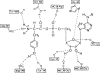
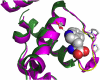
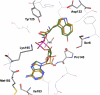
The site-selective encoding of noncanonical amino acids (NAAs) is a powerful technique for the installation of novel chemical functional groups in proteins. This is often achieved by recoding a stop codon and requires two additional components: an evolved aminoacyl tRNA synthetase (AARS) and a cognate tRNA. Analysis of the most successful AARSs reveals common characteristics. The highest fidelity NAA systems derived from the Methanocaldococcus jannaschii tyrosyl AARS feature specific mutations to two residues reported to interact with the hydroxyl group of the substrate tyrosine. We demonstrate that the restoration of just one of these determinants for amino acid specificity results in the loss of fidelity as the evolved AARSs become noticeably promiscuous. These results offer a partial explanation of a recently retracted strategy for the synthesis of glycoproteins. Similarly, we reinvestigated a tryptophanyl AARS reported to allow the site-selective incorporation of 5-hydroxy tryptophan within mammalian cells. In multiple experiments, the enzyme displayed elements of promiscuity despite its previous characterization as a high fidelity enzyme. Given the many similarities of the TyrRSs and TrpRSs reevaluated here, our findings can be largely combined, and in doing so they reinforce the long-established central dogma regarding the molecular basis by which these enzymes contribute to the fidelity of translation. Thus, our view is that the central claims of fidelity reported in several NAA systems remain unproven and unprecedented.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
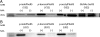

Similar articles
-
Exploring the substrate range of wild-type aminoacyl-tRNA synthetases.Chembiochem. 2014 Aug 18;15(12):1805-1809. doi: 10.1002/cbic.201402083. Epub 2014 May 30. Chembiochem. 2014. PMID: 24890918 Free PMC article.
-
Structural basis for orthogonal tRNA specificities of tyrosyl-tRNA synthetases for genetic code expansion.Nat Struct Biol. 2003 Jun;10(6):425-32. doi: 10.1038/nsb934. Nat Struct Biol. 2003. PMID: 12754495
-
Functional replacement of the endogenous tyrosyl-tRNA synthetase-tRNATyr pair by the archaeal tyrosine pair in Escherichia coli for genetic code expansion.Nucleic Acids Res. 2010 Jun;38(11):3682-91. doi: 10.1093/nar/gkq080. Epub 2010 Feb 16. Nucleic Acids Res. 2010. PMID: 20159998 Free PMC article.
-
Engineering the Genetic Code in Cells and Animals: Biological Considerations and Impacts.Acc Chem Res. 2017 Nov 21;50(11):2767-2775. doi: 10.1021/acs.accounts.7b00376. Epub 2017 Oct 6. Acc Chem Res. 2017. PMID: 28984438 Free PMC article. Review.
-
Plasticity and Constraints of tRNA Aminoacylation Define Directed Evolution of Aminoacyl-tRNA Synthetases.Int J Mol Sci. 2019 May 9;20(9):2294. doi: 10.3390/ijms20092294. Int J Mol Sci. 2019. PMID: 31075874 Free PMC article. Review.
Cited by
-
An orthogonalized platform for genetic code expansion in both bacteria and eukaryotes.Nat Chem Biol. 2017 Apr;13(4):446-450. doi: 10.1038/nchembio.2312. Epub 2017 Feb 13. Nat Chem Biol. 2017. PMID: 28192410
-
Efficient synthesis and in vivo incorporation of acridon-2-ylalanine, a fluorescent amino acid for lifetime and Förster resonance energy transfer/luminescence resonance energy transfer studies.J Am Chem Soc. 2013 Dec 18;135(50):18806-14. doi: 10.1021/ja403247j. Epub 2013 Dec 4. J Am Chem Soc. 2013. PMID: 24303933 Free PMC article.
-
Engineering posttranslational proofreading to discriminate nonstandard amino acids.Proc Natl Acad Sci U S A. 2018 Jan 16;115(3):619-624. doi: 10.1073/pnas.1715137115. Epub 2018 Jan 4. Proc Natl Acad Sci U S A. 2018. PMID: 29301968 Free PMC article.
-
Computational Aminoacyl-tRNA Synthetase Library Design for Photocaged Tyrosine.Int J Mol Sci. 2019 May 11;20(9):2343. doi: 10.3390/ijms20092343. Int J Mol Sci. 2019. PMID: 31083552 Free PMC article.
-
Site-specific dual encoding and labeling of proteins via genetic code expansion.Cell Chem Biol. 2023 Apr 20;30(4):343-361. doi: 10.1016/j.chembiol.2023.03.004. Epub 2023 Mar 27. Cell Chem Biol. 2023. PMID: 36977415 Free PMC article. Review.
References
-
- Budisa N. Engineering the Genetic Code. Weinheim, Germany: Wiley; 2006.
-
- Delarue M, Moras D. The aminoacyl-tRNA synthetase family: Modules at work. Bioessays. 1993;15:675–687. - PubMed
-
- Carter CW., Jr Cognition, mechanism, and evolutionary relationships in aminoacyl-tRNA synthetases. Annu Rev Biochem. 1993;62:715–748. - PubMed
-
- Ibba M, Soll D. Aminoacyl-tRNA synthesis. Annu Rev Biochem. 2000;69:617–650. - PubMed
-
- Silvian LF, Wang J, Steitz TA. Insights into editing from an ile-tRNA synthetase structure with tRNAile and mupirocin. Science. 1999;285:1074–1077. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

