Cytokinin regulates the activity of reproductive meristems, flower organ size, ovule formation, and thus seed yield in Arabidopsis thaliana
- PMID: 21224426
- PMCID: PMC3051259
- DOI: 10.1105/tpc.110.079079
Cytokinin regulates the activity of reproductive meristems, flower organ size, ovule formation, and thus seed yield in Arabidopsis thaliana
Abstract
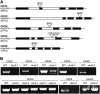
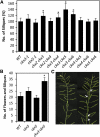
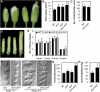
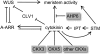
The size and activity of the shoot apical meristem is regulated by transcription factors and low molecular mass signals, including the plant hormone cytokinin. The cytokinin status of the meristem depends on different factors, including metabolic degradation of the hormone, which is catalyzed by cytokinin oxidase/dehydrogenase (CKX) enzymes. Here, we show that CKX3 and CKX5 regulate the activity of the reproductive meristems of Arabidopsis thaliana. CKX3 is expressed in the central WUSCHEL (WUS) domain, while CKX5 shows a broader meristematic expression. ckx3 ckx5 double mutants form larger inflorescence and floral meristems. An increased size of the WUS domain and enhanced primordia formation indicate a dual function for cytokinin in defining the stem cell niche and delaying cellular differentiation. Consistent with this, mutation of a negative regulator gene of cytokinin signaling, ARABIDOPSIS HISTIDINE PHOSPHOTRANSFER PROTEIN 6, which is expressed at the meristem flanks, caused a further delay of differentiation. Terminal cellular differentiation was also retarded in ckx3 ckx5 flowers, which formed more cells and became larger, corroborating the role of cytokinin in regulating flower organ size. Furthermore, higher activity of the ckx3 ckx5 placenta tissue established supernumerary ovules leading to an increased seed set per silique. Together, the results underpin the important role of cytokinin in reproductive development. The increased cytokinin content caused an ~55% increase in seed yield, highlighting the relevance of sink strength as a yield factor.
Figures





References
-
- Aizen M.A., Garibaldi L.A., Cunningham S.A., Klein A.M. (2008). Long-term global trends in crop yield and production reveal no current pollination shortage but increasing pollinator dependency. Curr. Biol. 18: 1572–1575 - PubMed
-
- Alonso J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 - PubMed
-
- Anastasiou E., Kenz S., Gerstung M., MacLean D., Timmer J., Fleck C., Lenhard M. (2007). Control of plant organ size by KLUH/CYP78A5-dependent intercellular signaling. Dev. Cell 13: 843–856 - PubMed
-
- Ashikari M., Sakakibara H., Lin S., Yamamoto T., Takashi T., Nishimura A., Angeles E.R., Qian Q., Kitano H., Matsuoka M. (2005). Cytokinin oxidase regulates rice grain production. Science 309: 741–745 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

