Effect of direct-to-consumer genomewide profiling to assess disease risk
- PMID: 21226570
- PMCID: PMC3786730
- DOI: 10.1056/NEJMoa1011893
Effect of direct-to-consumer genomewide profiling to assess disease risk
Abstract
Background: The use of direct-to-consumer genomewide profiling to assess disease risk is controversial, and little is known about the effect of this technology on consumers. We examined the psychological, behavioral, and clinical effects of risk scanning with the Navigenics Health Compass, a commercially available test of uncertain clinical validity and utility.
Methods: We recruited subjects from health and technology companies who elected to purchase the Health Compass at a discounted rate. Subjects reported any changes in symptoms of anxiety, intake of dietary fat, and exercise behavior at a mean (±SD) of 5.6±2.4 months after testing, as compared with baseline, along with any test-related distress and the use of health-screening tests.
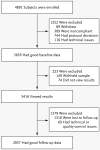
Results: From a cohort of 3639 enrolled subjects, 2037 completed follow-up. Primary analyses showed no significant differences between baseline and follow-up in anxiety symptoms (P=0.80), dietary fat intake (P=0.89), or exercise behavior (P=0.61). Secondary analyses revealed that test-related distress was positively correlated with the average estimated lifetime risk among all the assessed conditions (β=0.117, P<0.001). However, 90.3% of subjects who completed follow-up had scores indicating no test-related distress. There was no significant increase in the rate of use of screening tests associated with genomewide profiling, most of which are not considered appropriate for screening asymptomatic persons in any case.
Conclusions: In a selected sample of subjects who completed follow-up after undergoing consumer genomewide testing, such testing did not result in any measurable short-term changes in psychological health, diet or exercise behavior, or use of screening tests. Potential effects of this type of genetic testing on the population at large are not known. (Funded by the National Institutes of Health and Scripps Health.).
Figures
Comment in
-
Direct-to-consumer genomewide profiling.N Engl J Med. 2011 May 26;364(21):2074-5; author reply 2075. doi: 10.1056/NEJMc1103048. N Engl J Med. 2011. PMID: 21612491 No abstract available.
-
Direct-to-consumer genomewide profiling.N Engl J Med. 2011 May 26;364(21):2074; author reply 2075. doi: 10.1056/NEJMc1103048. N Engl J Med. 2011. PMID: 21612492 No abstract available.
References
-
- deCODEme home page. http://www.decodeme.com.
-
- Navigenics home page. http://www.navigenics.com.
-
- Pathway Genomics home page. http://www.pathway.com.
-
- 23andMe home page. https://www.23andme.com.
-
- Botkin JR, Smith KR, Croyle RT, et al. Genetic testing for a BRCA1 mutation: prophylactic surgery and screening behavior in women 2 years post testing. Am J Med Genet A. 2003;118A:201–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
