Iron-chelating therapy for transfusional iron overload
- PMID: 21226580
- PMCID: PMC3078566
- DOI: 10.1056/NEJMct1004810
Iron-chelating therapy for transfusional iron overload
Abstract
A 16-year-old boy with sickle cell anemia undergoes routine screening with transcranial Doppler ultrasonography to assess the risk of stroke. This examination shows an abnormally elevated blood-flow velocity in the middle cerebral artery. The hemoglobin level is 7.2 g per deciliter, the reticulocyte count is 12.5%, and the fetal hemoglobin level is 8.0%. Long-term treatment with red-cell transfusion is initiated to prevent stroke. A hematologist recommends prophylactic iron-chelating therapy.
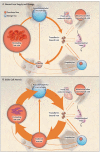
Figures

Comment in
-
Iron-chelating therapy for transfusional iron overload.N Engl J Med. 2011 Apr 14;364(15):1476-7; author reply 1477. doi: 10.1056/NEJMc1101838. N Engl J Med. 2011. PMID: 21488786 No abstract available.
-
Iron-chelating therapy for transfusional iron overload.N Engl J Med. 2011 Apr 14;364(15):1476; author reply 1477. doi: 10.1056/NEJMc1101838. N Engl J Med. 2011. PMID: 21488787 No abstract available.
-
Iron-chelating therapy for transfusional iron overload.N Engl J Med. 2011 Apr 14;364(15):1475-6; author reply 1477. doi: 10.1056/NEJMc1101838. N Engl J Med. 2011. PMID: 21488788 No abstract available.
References
-
- Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339:5–11. - PubMed
-
- Vichinsky EP, MacKlin EA, Waye JS, Lorey F, Olivieri NF. Changes in the epidemiology of thalassemia in North America: a new minority disease. Pediatrics. 2005;116(6):e818–e825. - PubMed
-
- Weatherall DJ, Clegg JB. The thalassemia syndromes. 4th ed. Blackwell Science; Oxford, England: 2001.
-
- Darbari DS, Kple-Faget P, Kwagyan J, Rana S, Gordeuk VR, Castro O. Circumstances of death in adult sickle cell disease patients. Am J Hematol. 2006;81:858–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
