Optimized allele-specific real-time PCR assays for the detection of common mutations in KRAS and BRAF
- PMID: 21227391
- PMCID: PMC3070579
- DOI: 10.1016/j.jmoldx.2010.11.007
Optimized allele-specific real-time PCR assays for the detection of common mutations in KRAS and BRAF
Abstract
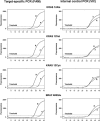
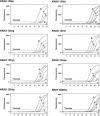
Mutations in the oncogenes KRAS and BRAF have been identified as prognostic factors in patients with colorectal diseases and as predictors of negative outcome in epidermal growth factor receptor-targeted therapies. Therefore, accurate mutation detection in both genes, KRAS and BRAF, is of increasing clinical relevance. We aimed at optimizing allele-specific real-time PCR assays for the detection of common mutations in KRAS and the BRAF Val600Glu mutation using allele-specific PCR primers for allelic discrimination and probes (TaqMan) for quantification. Each reaction mix contains a co-amplified internal control to exclude false-negative results. Allele-specific real-time PCR assays were evaluated on plasmid model systems providing a mutation detection limit of 10 copies of mutant DNA in proportions as low as 1% of the total DNA. Furthermore, we analyzed 125 DNA samples prepared from archived, formalin-fixed, paraffin-embedded colorectal carcinomas and compared results with those obtained from direct-sequence analysis. All mutations determined by sequence analysis could be recovered by allele-specific PCR assays. In addition, allele-specific PCR assays clearly identified three additional samples affected by a mutation. We propose these allele-specific real-time PCR assays as a low-cost and fast diagnostic tool for accurate detection of KRAS and BRAF mutations that can be applied to clinical samples.
Copyright © 2011 American Society for Investigative Pathology and the Association for Molecular Pathology. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Andreyev H.J., Norman A.R., Cunningham D., Oates J., Dix B.R., Iacopetta B.J., Young J., Walsh T., Ward R., Hawkins N., Beranek M., Jandik P., Benamouzig R., Jullian E., Laurent-Puig P., Olschwang S., Muller O., Hoffmann I., Rabes H.M., Zietz C., Troungos C., Valavanis C., Yuen S.T., Ho J.W., Croke C.T., O'Donoghue D.P., Giaretti W., Rapallo A., Russo A., Bazan V., Tanaka M., Omura K., Azuma T., Ohkusa T., Fujimori T., Ono Y., Pauly M., Faber C., Glaesener R., de Goeij A.F., Arends J.W., Andersen S.N., Lovig T., Breivik J., Gaudernack G., Clausen O.P., De Angelis P.D., Meling G.I., Rognum T.O., Smith R., Goh H.S., Font A., Rosell R., Sun X.F., Zhang H., Benhattar J., Losi L., Lee J.Q., Wang S.T., Clarke P.A., Bell S., Quirke P., Bubb V.J., Piris J., Cruickshank N.R., Morton D., Fox J.C., Al Mulla F., Lees N., Hall C.N., Snary D., Wilkinson K., Dillon D., Costa J., Pricolo V.E., Finkelstein S.D., Thebo J.S., Senagore A.J., Halter S.A., Wadler S., Malik S., Krtolica K., Urosevic N. Kirsten ras mutations in patients with colorectal cancer: the “RASCAL II” study. Br J Cancer. 2001;85:692–696. - PMC - PubMed
-
- Bos J.L., Fearon E.R., Hamilton S.R., Verlaan-de Vries M., van Boom J.H., van der Eb A.J., Vogelstein B. Prevalence of ras gene mutations in human colorectal cancers. Nature. 1987;327:293–297. - PubMed
-
- Davies H., Bignell G.R., Cox C., Stephens P., Edkins S., Clegg S., Teague J., Woffendin H., Garnett M.J., Bottomley W., Davis N., Dicks E., Ewing R., Floyd Y., Gray K., Hall S., Hawes R., Hughes J., Kosmidou V., Menzies A., Mould C., Parker A., Stevens C., Watt S., Hooper S., Wilson R., Jayatilake H., Gusterson B.A., Cooper C., Shipley J., Hargrave D., Pritchard-Jones K., Maitland N., Chenevix-Trench G., Riggins G.J., Bigner D.D., Palmieri G., Cossu A., Flanagan A., Nicholson A., Ho J.W., Leung S.Y., Yuen S.T., Weber B.L., Seigler H.F., Darrow T.L., Paterson H., Marais R., Marshall C.J., Wooster R., Stratton M.R., Futreal P.A. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. - PubMed
-
- Laurent-Puig P., Cayre A., Manceau G., Buc E., Bachet J.B., Lecomte T., Rougier P., Lievre A., Landi B., Boige V., Ducreux M., Ychou M., Bibeau F., Bouche O., Reid J., Stone S., Penault-Llorca F. Analysis of PTEN, BRAF, and EGFR status in determining benefit from cetuximab therapy in wild-type KRAS metastatic colon cancer. J Clin Oncol. 2009;27:5924–5930. - PubMed
-
- Tol J., Nagtegaal I.D., Punt C.J. BRAF mutation in metastatic colorectal cancer. N Engl J Med. 2009;361:98–99. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

