EMMPRIN: a novel regulator of leukocyte transmigration into the CNS in multiple sclerosis and experimental autoimmune encephalomyelitis
- PMID: 21228176
- PMCID: PMC6623447
- DOI: 10.1523/JNEUROSCI.3659-10.2011
EMMPRIN: a novel regulator of leukocyte transmigration into the CNS in multiple sclerosis and experimental autoimmune encephalomyelitis
Abstract
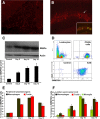
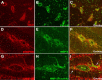
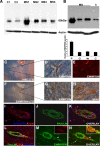
Extracellular matrix metalloproteinase inducer (EMMPRIN, CD147) is a member of the Ig superfamily, with various physiological roles including the induction of matrix metalloproteinases (MMPs), leukocyte activation, and tumor progression. In this study, we illustrate a novel involvement of EMMPRIN in multiple sclerosis (MS) and its animal model, experimental autoimmune encephalomyelitis (EAE). We found EMMPRIN levels to be upregulated on peripheral leukocytes before onset of EAE clinical signs and on infiltrating leukocytes and resident cells within the CNS in symptomatic mice. In EAE brain sections, EMMPRIN expression was localized with MMP-9 protein and activity. The increased EMMPRIN level was also characteristic of brain samples from MS subjects, particularly in plaque-containing areas. To evaluate the implications of elevated EMMPRIN levels, we treated EAE mice with an EMMPRIN function-blocking antibody and found reduced EAE clinical severity accompanied by decreased CNS parenchymal infiltration of leukocytes. Amelioration of EAE clinical signs by the anti-EMMPRIN antibody was critically dependent on its administration around the period of onset of clinical signs, which is typically associated with significant influx of leukocytes into the CNS. Moreover, the reduction in disease severity in anti-EMMPRIN-treated mice was associated with diminished MMP proteolytic activity at the glia limitans, the final barrier before parenchymal infiltration of leukocytes. Together, our results are the first to emphasize a role for EMMPRIN in MS and EAE, whereby EMMPRIN regulates leukocyte trafficking through increasing MMP activity. These results identify EMMPRIN as a novel therapeutic target in MS.
Figures



References
-
- Anthony DC, Ferguson B, Matyzak MK, Miller KM, Esiri MM, Perry VH. Differential matrix metalloproteinase expression in cases of multiple sclerosis and stroke. Neuropathol Appl Neurobiol. 1997;23:406–415. - PubMed
-
- Berditchevski F, Chang S, Bodorova J, Hemler ME. Generation of monoclonal antibodies to integrin-associated proteins. Evidence that alpha3beta1 complexes with EMMPRIN/basigin/OX47/M6. J Biol Chem. 1997;272:29174–29180. - PubMed
-
- Bernal F, Elias B, Hartung HP, Kieseier BC. Regulation of matrix metalloproteinases and their inhibitors by interferon-beta: a longitudinal study in multiple sclerosis patients. Mult Scler. 2009;15:721–727. - PubMed
-
- Biswas C, Zhang Y, DeCastro R, Guo H, Nakamura T, Kataoka H, Nabeshima K. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 1995;55:434–439. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
