ATP synthesis, mitochondrial function, and steroid biosynthesis in rodent primary and tumor Leydig cells
- PMID: 21228212
- PMCID: PMC3080423
- DOI: 10.1095/biolreprod.110.087460
ATP synthesis, mitochondrial function, and steroid biosynthesis in rodent primary and tumor Leydig cells
Abstract
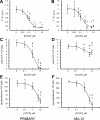
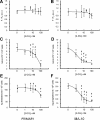
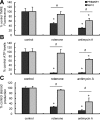
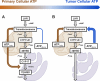
Previous studies in MA-10 tumor Leydig cells demonstrated that disruption of the mitochondrial electron-transport chain (ETC), membrane potential (ΔΨ(m)), or ATP synthesis independently inhibited steroidogenesis. In contrast, studies of primary Leydig cells indicated that the ETC, ΔΨ(m), and ATP synthesis cooperatively affected steroidogenesis. These results suggest significant differences between the two systems and call into question the extent to which results from tumor Leydig cells relate to primary cells. Thus, to further understand the similarities and differences between the two systems as well as the impact of ATP disruption on steroidogenesis, we performed comparative studies of MA-10 and primary Leydig cells under similar conditions of mitochondrial disruption. We show that mitochondrial ATP synthesis is critical for steroidogenesis in both primary and tumor Leydig cells. However, in striking contrast to primary cells, perturbation of ΔΨ(m) in MA-10 cells did not substantially decrease cellular ATP content, a perplexing finding because ΔΨ(m) powers the mitochondrial ATP synthase. Further studies revealed that a significant proportion of cellular ATP in MA-10 cells derives from glycolysis. In contrast, primary cells appear to be almost completely dependent on mitochondrial respiration for their energy provision. Inhibitor studies also suggested that the MA-10 ETC is impaired. This work underscores the importance of mitochondrial ATP for hormone-stimulated steroid production in both MA-10 and primary Leydig cells while indicating that caution must be exercised in extrapolating data from tumor cells to primary tissue.
Figures



References
-
- Miller WL. Steroidogenic acute regulatory protein (StAR), a novel mitochondrial cholesterol transporter. Biochim Biophys Acta 2007; 1771: 663 676 - PubMed
-
- Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapère JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, Gavish M. Translocator protein (18 kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci 2006; 27: 402 409 - PubMed
-
- Liu J, Rone MB, Papadopoulos V. Protein-protein interactions mediate mitochondrial cholesterol transport and steroid biosynthesis. J Biol Chem 2006; 281: 38879 38893 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

