Effects of metformin on body weight and body composition in obese insulin-resistant children: a randomized clinical trial
- PMID: 21228310
- PMCID: PMC3028347
- DOI: 10.2337/db10-1185
Effects of metformin on body weight and body composition in obese insulin-resistant children: a randomized clinical trial
Abstract
Objective: Metformin can decrease adiposity and ameliorate obesity-related comorbid conditions, including abnormalities in glucose homeostasis in adolescents, but there are few data evaluating the efficacy of metformin among younger children. Our objective was to determine whether metformin treatment causes weight loss and improves obesity-related comorbidities in obese children, who are insulin-resistant.
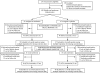
Research design and methods: This study was a randomized double-blind placebo-controlled trial consisting of 100 severely obese (mean BMI 34.6 ± 6.6 kg/m(2)) insulin-resistant children aged 6-12 years, randomized to 1,000 mg metformin (n = 53) or placebo (n = 47) twice daily for 6 months, followed by open-label metformin treatment for 6 months. All children and their parents participated in a monthly dietitian-administered weight-reduction program.
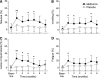
Results: Eighty-five percent completed the 6-month randomized phase. Children prescribed metformin had significantly greater decreases in BMI (difference -1.09 kg/m(2), CI -1.87 to -0.31, P = 0.006), body weight (difference -3.38 kg, CI -5.2 to -1.57, P < 0.001), BMI Z score (difference between metformin and placebo groups -0.07, CI -0.12 to -0.01, P = 0.02), and fat mass (difference -1.40 kg, CI -2.74 to -0.06, P = 0.04). Fasting plasma glucose (P = 0.007) and homeostasis model assessment (HOMA) insulin resistance index (P = 0.006) also improved more in metformin-treated children than in placebo-treated children. Gastrointestinal symptoms were significantly more prevalent in metformin-treated children, which limited maximal tolerated dosage in 17%. During the 6-month open-label phase, children treated previously with placebo decreased their BMI Z score; those treated continuously with metformin did not significantly change BMI Z score further.
Conclusions: Metformin had modest but favorable effects on body weight, body composition, and glucose homeostasis in obese insulin-resistant children participating in a low-intensity weight-reduction program.
Trial registration: ClinicalTrials.gov NCT00005669.
Figures

References
-
- Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007-2008. JAMA 2010;303:242–249 - PubMed
-
- Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 2004;350:2362–2374 - PubMed
-
- Lee JW, Lee DC, Im JA, Shim JY, Kim SM, Lee HR. Insulin resistance is associated with arterial stiffness independent of obesity in male adolescents. Hypertens Res 2007;30:5–11 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

