Adverse and protective influences of adenosine on the newborn and embryo: implications for preterm white matter injury and embryo protection
- PMID: 21228731
- PMCID: PMC3100210
- DOI: 10.1203/PDR.0b013e31820efbcf
Adverse and protective influences of adenosine on the newborn and embryo: implications for preterm white matter injury and embryo protection
Abstract
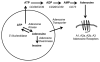
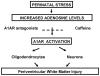
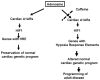
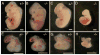
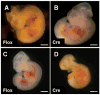
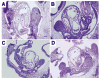
Few signaling molecules have the potential to influence the developing mammal as the nucleoside adenosine. Adenosine levels increase rapidly with tissue hypoxia and inflammation. Adenosine antagonists include the methylxanthines caffeine and theophylline. The receptors that transduce adenosine action are the A1, A2a, A2b, and A3 adenosine receptors (ARs). In the postnatal period, A1AR activation may contribute to white matter injury in the preterm infant by altering oligodendrocyte (OL) development. In models of perinatal brain injury, caffeine is neuroprotective against periventricular white matter injury (PWMI) and hypoxic-ischemic encephalopathy (HIE). Supporting the notion that blockade of adenosine action is of benefit in the premature infant, caffeine reduces the incidence of bronchopulmonary dysplasia and CP in clinical studies. In comparison with the adverse effects on the postnatal brain, adenosine acts via A1ARs to play an essential role in protecting the embryo from hypoxia. Embryo protective effects are blocked by caffeine, and caffeine intake during early pregnancy increases the risk of miscarriage and fetal growth retardation. Adenosine and adenosine antagonists play important modulatory roles during mammalian development. The protective and deleterious effects of adenosine depend on the time of exposure and target sites of action.
Figures





References
-
- Rivkees SA, Zhao Z, Porter G, Turner C. Influences of adenosine on the fetus and newborn. Mol Genet Metab. 2001;74:160–171. - PubMed
-
- Eckle T, Koeppen M, Eltzschig HK. Role of extracellular adenosine in acute lung injury. Physiology (Bethesda) 2009;24:298–306. - PubMed
-
- Eltzschig HK, Weissmuller T, Mager A, Eckle T. Nucleotide metabolism and cell-cell interactions. Methods Mol Biol. 2006;341:73–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

