Deconstructing the "resting" state: exploring the temporal dynamics of frontal alpha asymmetry as an endophenotype for depression
- PMID: 21228910
- PMCID: PMC3017362
- DOI: 10.3389/fnhum.2010.00232
Deconstructing the "resting" state: exploring the temporal dynamics of frontal alpha asymmetry as an endophenotype for depression
Abstract
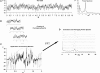
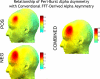
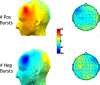
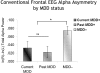
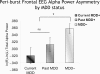
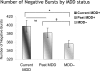
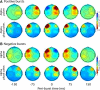
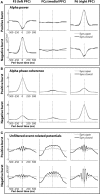
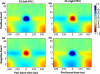
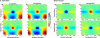
Asymmetry in frontal electrocortical alpha-band (8-13 Hz) activity recorded during resting situations (i.e., in absence of a specific task) has been investigated in relation to emotion and depression for over 30 years. This asymmetry reflects an aspect of endogenous cortical dynamics that is stable over repeated measurements and that may serve as an endophenotype for mood or other psychiatric disorders. In nearly all of this research, EEG activity is averaged across several minutes, obscuring transient dynamics that unfold on the scale of milliseconds to seconds. Such dynamic states may ultimately have greater value in linking brain activity to surface EEG asymmetry, thus improving its status as an endophenotype for depression. Here we introduce novel metrics for characterizing frontal alpha asymmetry that provide a more in-depth neurodynamical understanding of recurrent endogenous cortical processes during the resting-state. The metrics are based on transient "bursts" of asymmetry that occur frequently during the resting-state. In a sample of 306 young adults, 143 with a lifetime diagnosis of major depressive disorder (62 currently symptomatic), three questions were addressed: (1) How do novel peri-burst metrics of dynamic asymmetry compare to conventional fast-Fourier transform-based metrics? (2) Do peri-burst metrics adequately differentiate depressed from non-depressed participants? and, (3) what EEG dynamics surround the asymmetry bursts? Peri-burst metrics correlated with traditional measures of asymmetry, and were sensitive to both current and past episodes of major depression. Moreover, asymmetry bursts were characterized by a transient lateralized alpha suppression that is highly consistent in phase across bursts, and a concurrent contralateral transient alpha enhancement that is less tightly phase-locked across bursts. This approach opens new possibilities for investigating rapid cortical dynamics during resting-state EEG.
Keywords: EEG asymmetry; EEG dynamics; depression; endophenotype; resting-state.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

