Emerging synthetic approaches for protein-polymer conjugations
- PMID: 21229146
- PMCID: PMC3066092
- DOI: 10.1039/c0cc04062b
Emerging synthetic approaches for protein-polymer conjugations
Abstract
Protein-polymer conjugates are important in diverse fields including drug delivery, biotechnology, and nanotechnology. This feature article highlights recent advances in the synthesis and application of protein-polymer conjugates by controlled radical polymerization techniques. Special emphasis on new applications of the materials, particularly in biomedicine, is provided.
Figures
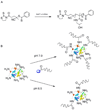
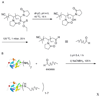

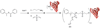


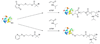


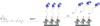




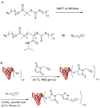

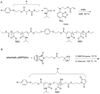
References
-
- Duncan R. Nat. Rev. Drug Discovery. 2003;2:347–360. - PubMed
-
- Jain A, Jain SK. Crit. Rev. Ther. Drug Carrier Syst. 2008;25:403–447. - PubMed
-
- Nucci ML, Shorr R, Abuchowski A. Adv. Drug Delivery Rev. 1991;6:133–151.
-
- Abuchowski A, Davis FF. Biochim. Biophys. Acta. 1979;578:41–46. - PubMed
-
- Hadley KB, Sato PH. Enzyme. 1989;42:225–234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

