Factors governing fibrillogenesis of polypeptide chains revealed by lattice models
- PMID: 21231356
- PMCID: PMC11298782
- DOI: 10.1103/PhysRevLett.105.218101
Factors governing fibrillogenesis of polypeptide chains revealed by lattice models
Abstract
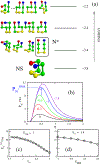
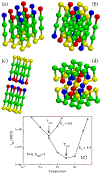
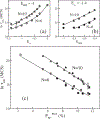
Using lattice models we explore the factors that determine the tendencies of polypeptide chains to aggregate by exhaustively sampling the sequence and conformational space. The morphologies of the fibril-like structures and the time scales (τ(fib)) for their formation depend on a balance between hydrophobic and Coulomb interactions. The extent of population of an ensemble of N* structures, which are fibril-prone structures in the spectrum of conformations of an isolated protein, is the major determinant of τ(fib). This observation is used to determine the aggregating sequences by exhaustively exploring the sequence space, thus providing a basis for genome wide search of fragments that are aggregation prone.
Figures
References
-
- (a) Otzen DE, Kristensen O, and Oliveberg M, Proc. Natl. Acad. Sci. U.S.A. 97, 9907 (2000); - PMC - PubMed
- (b) Massi F, Klimov D, Thirumalai D, and Straub JE, Prot. Sci. 11, 1639 (2002); - PMC - PubMed
- (c) Chiti F, Calamai M, Taddei N, Stefani M, Ramponi G, and Dobson CM, Proc. Natl. Acad. Sci. U.S.A. 99, 16419 (2002); - PMC - PubMed
- (d) West MW, Wang WX, Patterson J, Mancias JD, Beasley JR, and Hecht MH, Proc. Natl. Acad. Sci. U.S.A. 96, 11211 (1999); - PMC - PubMed
- (e) Kallberg Y, Gustafsson M, Persson B, Thyberg J, and Johansson J, J. Biol. Chem. 276, 12945 (2001); - PubMed
- (f) Dima RI and Thirumalai D, Biophys. J. 83, 1268 (2002); - PMC - PubMed
- (g) Gazit E, FASEB monographs 16, 77 (2002). - PubMed
-
- (a) Klimov DK and Thirumalai D, Structure 11, 295 (2003); - PubMed
- (b) Bellesia G and Shea JE, Biophys. J. 96, 875 (2009); - PMC - PubMed
- (c) de la Paz ML, de Mori GMS, Serrano L, and Colombo G, J. Mol. Biol 349, 583 (2005); - PubMed
- (d) Li DW, Mohanty S, Irback A, and Huo SH, PLoS Comput. Biol 4, e1000238 (2008). - PMC - PubMed
-
- (a) Klimov DK and Thirumalai D, J. Chem. Phys. 109, 4119 (1998);
- (b) Shakhnovich E, Chem. Rev 106, 1559 (2006); - PMC - PubMed
- (c) Gupta P, Hall CK, and Voegler AC, Protein Sci 7, 2642 (1998); - PMC - PubMed
- (d) Dima RI and Thirumalai D, Protein Sci 11, 1036 (2002); - PMC - PubMed
- (e) Maiti M, Rao M, and Sastry S, Eur. Phys. J. E 32, 217 (2010); - PubMed
- Cieplak M, Hoang TX, and Li MS, Phys. Rev. Lett 83, 1684 (1999);
- Li MS, Klimov DK, and Thirumalai D, Phys. Rev. Lett 93, 268107 (2004); - PubMed
- Li MS and Cieplak M, Phys. Rev. E 59, 970 (1999);
- Kouza M, Li MS, O’Brien EP, Hu C-K, and Thirumalai D, J. Phys. Chem. A 110, 671 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
