Bacterial flavohemoglobin: a molecular tool to probe mammalian nitric oxide biology
- PMID: 21231921
- PMCID: PMC3096140
- DOI: 10.2144/000113586
Bacterial flavohemoglobin: a molecular tool to probe mammalian nitric oxide biology
Abstract
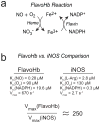
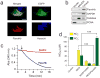
A wide range of mammalian signaling and stress pathways are mediated by nitric oxide (NO), which is synthesized in vivo by the nitric oxide synthase (NOS) family of enzymes. Experimental manipulations of NO are frequently achieved by either inhibition or activation of endogenous NOS or via providing exogenous NO sources. On the contrary, many microbes consume NO via flavohemoglobin (FlavoHb), a highly efficient NO-dioxygenase that protects from nitrosative stress. Here we report a novel resource for studying NO in mammalian cells by heterologously expressing Escherichia coli FlavoHb within a lentiviral delivery system. This technique boosts endogenous cellular consumption of NO, thus providing a simple and efficacious approach to studying mammalian NO biology that can be employed as both a primary experimental and confirmatory tool.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Figures

Similar articles
-
Protection from nitrosative stress: a central role for microbial flavohemoglobin.Free Radic Biol Med. 2012 May 1;52(9):1620-33. doi: 10.1016/j.freeradbiomed.2012.01.028. Epub 2012 Feb 6. Free Radic Biol Med. 2012. PMID: 22343413 Review.
-
Flavohemoglobin detoxifies nitric oxide in aerobic, but not anaerobic, Escherichia coli. Evidence for a novel inducible anaerobic nitric oxide-scavenging activity.J Biol Chem. 2002 Mar 8;277(10):8166-71. doi: 10.1074/jbc.M110470200. Epub 2001 Dec 18. J Biol Chem. 2002. PMID: 11751864
-
Structural studies on flavohemoglobins.Methods Enzymol. 2008;436:187-202. doi: 10.1016/S0076-6879(08)36010-8. Methods Enzymol. 2008. PMID: 18237633
-
Assay and characterization of the NO dioxygenase activity of flavohemoglobins.Methods Enzymol. 2008;436:217-37. doi: 10.1016/S0076-6879(08)36012-1. Methods Enzymol. 2008. PMID: 18237635
-
Nitric oxide dioxygenase function and mechanism of flavohemoglobin, hemoglobin, myoglobin and their associated reductases.J Inorg Biochem. 2005 Jan;99(1):247-66. doi: 10.1016/j.jinorgbio.2004.10.003. J Inorg Biochem. 2005. PMID: 15598505 Review.
Cited by
-
Detection and quantification of nitric oxide-derived oxidants in biological systems.J Biol Chem. 2019 Oct 4;294(40):14776-14802. doi: 10.1074/jbc.REV119.006136. Epub 2019 Aug 12. J Biol Chem. 2019. PMID: 31409645 Free PMC article. Review.
-
Strategies among phytoplankton in response to alleviation of nutrient stress in a subtropical gyre.ISME J. 2019 Dec;13(12):2984-2997. doi: 10.1038/s41396-019-0489-6. Epub 2019 Aug 22. ISME J. 2019. PMID: 31439897 Free PMC article.
-
Hemoglobin: a nitric-oxide dioxygenase.Scientifica (Cairo). 2012;2012:683729. doi: 10.6064/2012/683729. Epub 2012 Dec 19. Scientifica (Cairo). 2012. PMID: 24278729 Free PMC article. Review.
-
Enzymatic Mechanisms Involved in Evasion of Fungi to the Oxidative Stress: Focus on Scedosporium apiospermum.Mycopathologia. 2018 Feb;183(1):227-239. doi: 10.1007/s11046-017-0160-6. Epub 2017 Jun 21. Mycopathologia. 2018. PMID: 28639066 Review.
-
The role of the unfolded protein response in arrhythmias.Channels (Austin). 2018;12(1):335-345. doi: 10.1080/19336950.2018.1516985. Channels (Austin). 2018. PMID: 30165782 Free PMC article. Review.
References
-
- Stamler JS, Singel DJ, Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992;258:1898–1902. - PubMed
-
- Jaffrey SR, Snyder SH. Nitric oxide: a neural messenger. Annu Rev Cell Dev Biol. 1995;11:417–440. - PubMed
-
- Spiro S. Regulators of bacterial responses to nitric oxide. FEMS Microbiol Rev. 2007;31:193–211. - PubMed
-
- Moreau M, Lindermayr C, Durner J, Klessig DF. NO synthesis and signaling in plants--where do we stand? Physiol Plant. 2010;138:372–383. - PubMed
-
- Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol. 2004;2:820–832. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
