Vascular responses to 8-nitro-cyclic GMP in non-diabetic and diabetic mice
- PMID: 21232030
- PMCID: PMC3081129
- DOI: 10.1111/j.1476-5381.2011.01201.x
Vascular responses to 8-nitro-cyclic GMP in non-diabetic and diabetic mice
Abstract
Background and purpose: 8-Nitroguanosine 3',5'-cyclic monophosphate (8-nitro-cGMP), formed nitric oxide (NO)-dependently, is a physiological second messenger, yet little is known about its role in the pathophysiology of vascular diseases. To study the pharmacological activity of 8-nitro-cGMP in diabetic mice, we compared its effects on vascular reactivity of aortas from non-diabetic and diabetic mice.
Experimental approach: Vascular tension recording was performed in thoracic aortic rings from wild-type (C57BL/6), non-diabetic db/+ and obese/diabetic db/db mice. Endothelial NO synthase (eNOS) uncoupling and superoxide were tested by Western blot and dihydroethidium fluorescence respectively.
Key results: 8-Nitro-cGMP, at concentrations up to 10 µM, enhanced phenylephrine-induced contractions in aortas from C57BL/6 and db/+ mice, but not from db/db mice. This enhancement was not observed with 8-bromo-cGMP. Pretreatment of aortas from C57BL/6 and db/+ mice with l-NAME (100 µM), superoxide dismutase (100 U·mL(-1) ) or tiron (1 mM), abolished 8-nitro-cGMP-induced enhancement of the phenylephrine contraction. In 8-nitro-cGMP (10 µM)-treated C57BL/6 aortas, eNOS dimer/monomer ratio was significantly decreased and vascular superoxide production increased, suggesting that 8-nitro-cGMP-induced superoxide production via eNOS uncoupling may mediate the enhancement of the phenylephrine contraction. At higher concentrations (>10 µM), 8-nitro-cGMP produced relaxation of the phenylephrine-contracted aortas from C57BL/6, db/+ and db/db mice. The 8-nitro-cGMP-induced relaxation in db/db mouse aortas was found to be resistant to a phosphodiesterase 5 inhibitor, zaprinast (1 µM).
Conclusions and implications: The vasodilator effect of 8-nitro-cGMP may contribute to amelioration of the vascular endothelial dysfunction in diabetic mice, representing a novel pharmacological approach to prevent the complications associated with diabetes.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures


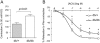


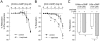

Similar articles
-
Endothelial nitric oxide synthase enhancer reduces oxidative stress and restores endothelial function in db/db mice.Cardiovasc Res. 2011 Nov 1;92(2):267-75. doi: 10.1093/cvr/cvr233. Epub 2011 Aug 29. Cardiovasc Res. 2011. PMID: 21875904
-
Gender differences in protein kinase G-mediated vasorelaxation of rat aorta.Clin Sci (Lond). 2001 May;100(5):473-9. Clin Sci (Lond). 2001. PMID: 11294687
-
Boldine improves endothelial function in diabetic db/db mice through inhibition of angiotensin II-mediated BMP4-oxidative stress cascade.Br J Pharmacol. 2013 Nov;170(6):1190-8. doi: 10.1111/bph.12350. Br J Pharmacol. 2013. PMID: 23992296 Free PMC article.
-
Histone demethylase LSD1 deficiency during high-salt diet is associated with enhanced vascular contraction, altered NO-cGMP relaxation pathway, and hypertension.Am J Physiol Heart Circ Physiol. 2011 Nov;301(5):H1862-71. doi: 10.1152/ajpheart.00513.2011. Epub 2011 Aug 26. Am J Physiol Heart Circ Physiol. 2011. PMID: 21873498 Free PMC article.
-
Formation, signaling functions, and metabolisms of nitrated cyclic nucleotide.Nitric Oxide. 2013 Nov 1;34:10-8. doi: 10.1016/j.niox.2013.04.004. Epub 2013 Apr 28. Nitric Oxide. 2013. PMID: 23632125 Review.
Cited by
-
New Therapeutic Implications of Endothelial Nitric Oxide Synthase (eNOS) Function/Dysfunction in Cardiovascular Disease.Int J Mol Sci. 2019 Jan 7;20(1):187. doi: 10.3390/ijms20010187. Int J Mol Sci. 2019. PMID: 30621010 Free PMC article. Review.
References
-
- Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48:1–9. - PubMed
-
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. - PubMed
-
- DeRubertis FR, Craven PA, Melhem MF, Salah EM. Attenuation of renal injury in db/db mice overexpressing superoxide dismutase: evidence for reduced superoxide-nitric oxide interaction. Diabetes. 2004;53:762–768. - PubMed
-
- Dong YF, Liu L, Kataoka K, Nakamura T, Fukuda M, Tokutomi Y, et al. Aliskiren prevents cardiovascular complications and pancreatic injury in a mouse model of obesity and type 2 diabetes. Diabetologia. 2010;53:180–191. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

