A humanized monoclonal antibody targeting the β7 integrin selectively blocks intestinal homing of T lymphocytes
- PMID: 21232034
- PMCID: PMC3081127
- DOI: 10.1111/j.1476-5381.2011.01205.x
A humanized monoclonal antibody targeting the β7 integrin selectively blocks intestinal homing of T lymphocytes
Abstract
Background and purpose: rhuMAb Beta7 is a humanized anti-human β7 monoclonal antibody currently in phase I in inflammatory bowel disease. rhuMAb Beta7 binds the β7 subunit of the integrins α4β7 and αEβ7, blocking interaction with their ligands. These integrins play key roles in immune cell homing to and retention in mucosal sites, and are associated with chronic inflammatory diseases of the gastrointestinal tract. The goal of this study was to evaluate the mucosal specificity of rhuMAb Beta7.
Experimental approach: We assessed the effect of murine anti-Beta7 on lymphocyte homing in mouse models of autoimmune disease. We also compared the effect of rhuMAb Beta7 on circulating mucosal-homing versus peripheral-homing T cells in naïve non-human primates.
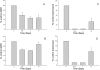
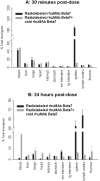
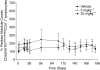
Key results: In cynomolgus monkeys, occupancy of β7 integrin receptors by rhuMAb Beta7 correlated with an increase in circulating β7(+) mucosal-homing lymphocytes, with no apparent effect on levels of circulating β7(-) peripheral-homing lymphocytes. rhuMAb Beta7 also inhibited lymphocyte homing to the inflamed colons of severe combined immunodeficient mice in CD45RB(high) CD4(+) T-cell transfer models. Consistent with a lack of effect on peripheral homing, in a mouse model of experimental autoimmune encephalomyelitis, anti-β7 treatment resulted in no amelioration of CNS inflammation.
Conclusions and implications: The results presented here suggest that rhuMAb Beta7 selectively blocks lymphocyte homing to the gastrointestinal tract without affecting lymphocyte trafficking to non-mucosal tissues. rhuMAb Beta7 provides a targeted therapeutic approach with the potential for a more attractive benefit:risk ratio than currently available inflammatory bowel disease therapies.
© 2011 Genentech, Inc.. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures
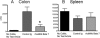





Similar articles
-
β7 Integrin Inhibition Can Increase Intestinal Inflammation by Impairing Homing of CD25hiFoxP3+ Regulatory T Cells.Cell Mol Gastroenterol Hepatol. 2020;9(3):369-385. doi: 10.1016/j.jcmgh.2019.10.012. Epub 2019 Nov 9. Cell Mol Gastroenterol Hepatol. 2020. PMID: 31707128 Free PMC article.
-
Beta7 integrins contribute to demyelinating disease of the central nervous system.J Neuroimmunol. 2000 Mar 1;103(2):146-52. doi: 10.1016/s0165-5728(99)00245-3. J Neuroimmunol. 2000. PMID: 10696909
-
Regulation and Role of αE Integrin and Gut Homing Integrins in Migration and Retention of Intestinal Lymphocytes during Inflammatory Bowel Disease.J Immunol. 2021 Nov 1;207(9):2245-2254. doi: 10.4049/jimmunol.2100220. Epub 2021 Sep 24. J Immunol. 2021. PMID: 34561227 Free PMC article.
-
Review article: nonclinical and clinical pharmacology, pharmacokinetics and pharmacodynamics of etrolizumab, an anti-β7 integrin therapy for inflammatory bowel disease.Aliment Pharmacol Ther. 2018 Jun;47(11):1440-1452. doi: 10.1111/apt.14631. Epub 2018 Mar 30. Aliment Pharmacol Ther. 2018. PMID: 29601644 Free PMC article. Review.
-
Role of beta7 integrins in intestinal lymphocyte homing and retention.Curr Mol Med. 2009 Sep;9(7):836-50. doi: 10.2174/156652409789105525. Curr Mol Med. 2009. PMID: 19860663 Free PMC article. Review.
Cited by
-
Targeting T-cell integrins in autoimmune and inflammatory diseases.Clin Exp Immunol. 2024 Jan 9;215(1):15-26. doi: 10.1093/cei/uxad093. Clin Exp Immunol. 2024. PMID: 37556361 Free PMC article. Review.
-
Clinical pharmacology of AMG 181, a gut-specific human anti-α4β7 monoclonal antibody, for treating inflammatory bowel diseases.Br J Clin Pharmacol. 2014 Dec;78(6):1315-33. doi: 10.1111/bcp.12418. Br J Clin Pharmacol. 2014. PMID: 24803302 Free PMC article. Clinical Trial.
-
Evaluation of assay interference and interpretation of CXCR4 receptor occupancy results in a preclinical study with MEDI3185, a fully human antibody to CXCR4.Cytometry B Clin Cytom. 2016 Mar;90(2):209-19. doi: 10.1002/cyto.b.21327. Epub 2015 Dec 15. Cytometry B Clin Cytom. 2016. PMID: 26384735 Free PMC article.
-
Emerging treatments for inflammatory bowel disease.Ther Adv Chronic Dis. 2020 Feb 5;11:2040622319899297. doi: 10.1177/2040622319899297. eCollection 2020. Ther Adv Chronic Dis. 2020. PMID: 32076497 Free PMC article. Review.
-
Targeting Leukocyte Trafficking in Inflammatory Bowel Disease.BioDrugs. 2021 Sep;35(5):473-503. doi: 10.1007/s40259-021-00496-5. Epub 2021 Oct 6. BioDrugs. 2021. PMID: 34613592
References
-
- Allison M. PML problems loom for Rituxan. Nat Biotechnol. 2010;28:105–106. - PubMed
-
- Andrew DP, Berlin C, Honda S, Yoshino T, Hamann A, Holzmann B, et al. Distinct but overlapping epitopes are involved in α4β7-mediated adhesion to vascular cell adhesion molecule-1, mucosal adhesion molecule-1, fibronectin, and lymphocyte aggregation. J Immunol. 1994;153:3847–3861. - PubMed
-
- Baca M, Presta LG, O'Connor SJ, Wells JA. Antibody humanization using monovalent phage display. J Biol Chem. 1997;272:10678–10684. - PubMed
-
- Baker DE. Natalizumab: overview of its pharmacology and safety. Rev Gastroenterol Disord. 2007;7:38–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

