Different sensitivities of rat skeletal muscles and brain to novel anti-cholinesterase agents, alkylammonium derivatives of 6-methyluracil (ADEMS)
- PMID: 21232040
- PMCID: PMC3111676
- DOI: 10.1111/j.1476-5381.2011.01211.x
Different sensitivities of rat skeletal muscles and brain to novel anti-cholinesterase agents, alkylammonium derivatives of 6-methyluracil (ADEMS)
Abstract
Background and purpose: The rat respiratory muscle diaphragm has markedly lower sensitivity than the locomotor muscle extensor digitorum longus (EDL) to the new acetylcholinesterase (AChE) inhibitors, alkylammonium derivatives of 6-methyluracil (ADEMS). This study evaluated several possible reasons for differing sensitivity between the diaphragm and limb muscles and between the muscles and the brain.
Experimental approach: Increased amplitude and prolonged decay time of miniature endplate currents were used to assess anti-cholinesterase activity in muscles. In hippocampal slices, induction of synchronous network activity was used to follow cholinesterase inhibition. The inhibitor sensitivities of purified AChE from the EDL and brain were also estimated.
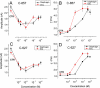
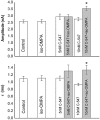
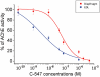
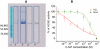
Key results: The intermuscular difference in sensitivity to ADEMS is partly explained caused by a higher level of mRNA and activity of 1,3-bis[5(diethyl-o-nitrobenzylammonium)pentyl]-6-methyluracildibromide (C-547)-resistant BuChE in the diaphragm. Moreover, diaphragm AChE was more than 20 times less sensitive to C-547 than that from the EDL. Sensitivity of the EDL to C-547 dramatically decreased after treadmill exercises that increased the amount of PRiMA AChE(G4), but not ColQ AChE(A12) molecular forms. The A12 form present in muscles appeared more sensitive to C-547. The main form of AChE in brain, PRiMA AChE(G4), was apparently less sensitive because brain cholinesterase activity was almost three orders of magnitude more resistant to C-547 than that of the EDL.
Conclusions and implications: Our findings suggest that ADEMS compounds could be used for the selective inhibition of AChEs and as potential therapeutic tools.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures




Similar articles
-
Different sensitivity of miniature endplate currents in rat external and internal intercostal muscles to the acetylcholinesterase inhibitor C-547 as compared with diaphragm and extensor digitorum longus.Physiol Res. 2009;58(1):149-153. doi: 10.33549/physiolres.931698. Physiol Res. 2009. PMID: 19331513
-
Different sensitivity of miniature endplate currents of the rat extensor digitorum longus, soleus and diaphragm muscles to a novel acetylcholinesterase inhibitor C-547.Physiol Res. 2006;55(5):585-589. doi: 10.33549/physiolres.930980. Epub 2006 Jun 22. Physiol Res. 2006. PMID: 16792471
-
Effect of tissue-specific acetylcholinesterase inhibitor C-547 on α3β4 and αβεδ acetylcholine receptors in COS cells.Eur J Pharmacol. 2012 Aug 5;688(1-3):22-6. doi: 10.1016/j.ejphar.2012.05.010. Epub 2012 May 23. Eur J Pharmacol. 2012. PMID: 22634638
-
Specific inhibitory effects of the alkylammonium derivative 6-methyluracil on acetylcholinesterase of smooth and striated muscles in rats.Dokl Biol Sci. 2013 Mar;449:82-4. doi: 10.1134/S0012496613020099. Epub 2013 May 8. Dokl Biol Sci. 2013. PMID: 23652433 No abstract available.
-
The effect of continuous pyridostigmine administration on functional (A12) acetylcholinesterase activity in guinea-pig muscles.Neurotoxicology. 2001 Dec;22(6):787-93. doi: 10.1016/s0161-813x(01)00061-4. Neurotoxicology. 2001. PMID: 11829412
Cited by
-
From Frog Muscle to Brain Neurons: Joys and Sorrows in Neuroscience.Physiol Res. 2024 Aug 30;73(S1):S83-S103. doi: 10.33549/physiolres.935414. Epub 2024 Jul 2. Physiol Res. 2024. PMID: 38957950 Free PMC article. Review.
-
Slow-binding reversible inhibitor of acetylcholinesterase with long-lasting action for prophylaxis of organophosphate poisoning.Sci Rep. 2020 Oct 6;10(1):16611. doi: 10.1038/s41598-020-73822-6. Sci Rep. 2020. PMID: 33024231 Free PMC article.
-
Autoregulation of Acetylcholine Release and Micro-Pharmacodynamic Mechanisms at Neuromuscular Junction: Selective Acetylcholinesterase Inhibitors for Therapy of Myasthenic Syndromes.Front Pharmacol. 2018 Jul 12;9:766. doi: 10.3389/fphar.2018.00766. eCollection 2018. Front Pharmacol. 2018. PMID: 30050445 Free PMC article. Review.
-
Crystallization and preliminary X-ray study of Vibrio cholerae uridine phosphorylase in complex with 6-methyluracil.Acta Crystallogr F Struct Biol Commun. 2014 Jan;70(Pt 1):60-3. doi: 10.1107/S2053230X13031877. Epub 2013 Dec 24. Acta Crystallogr F Struct Biol Commun. 2014. PMID: 24419619 Free PMC article.
-
Specific inhibition of acetylcholinesterase as an approach to decrease muscarinic side effects during myasthenia gravis treatment.Sci Rep. 2018 Jan 10;8(1):304. doi: 10.1038/s41598-017-18307-9. Sci Rep. 2018. PMID: 29321572 Free PMC article.
References
-
- Anikienko KA, Bychikhin EA, Reznik VS, Akamsin D, Galyametdinova IV. Compounds with the dioxopyrimidine cycle inhibit cholinesterases from different groups of animals. Chem Biol Interact. 2008;175:286–292. - PubMed
-
- Atack JR, Yu QS, Soncrant TT, Brossi A, Rapoport SI. Comparative inhibitory effects of various physostigmine analogs against acetyl- and butyrylcholinesterases. J Pharmacol Exp Ther. 1989;249:194–202. - PubMed
-
- Bon S, Ayon A, Leroy J, Massoulie J. Trimerization domain of the collagen tail of acetylcholinesterase. Neurochem Res. 2003;28:523–535. - PubMed
-
- Brenner T, Hamra-Amitay Y, Evron T, Boneva N, Seidman S, Soreq H. The role of readthrough acetylcholinesterase in the pathophysiology of myasthenia gravis. FASEB J. 2003;17:214–222. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

