Stable expression and functional characterization of a human nicotinic acetylcholine receptor with α6β2 properties: discovery of selective antagonists
- PMID: 21232042
- PMCID: PMC3087134
- DOI: 10.1111/j.1476-5381.2011.01213.x
Stable expression and functional characterization of a human nicotinic acetylcholine receptor with α6β2 properties: discovery of selective antagonists
Abstract
Background and purpose: Despite growing evidence that inhibition of α6β2-containing (α6β2*) nicotinic acetylcholine receptors (nAChRs) may be beneficial for the therapy of tobacco addiction, the lack of good sources of α6β2*-nAChRs has delayed the discovery of α6β2-selective antagonists. Our aim was to generate a cell line stably expressing functional nAChRs with α6β2 properties, to enable pharmacological characterization and the identification of novel α6β2-selective antagonists.
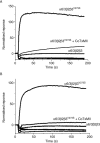
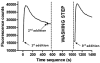
Experimental approach: Different combinations of the α6, β2, β3, chimeric α6/3 and mutant β3(V273S) subunits were transfected in human embryonic kidney cells and tested for activity in a fluorescent imaging plate reader assay. The pharmacology of rat immune-immobilized α6β2*-nAChRs was determined with ¹²⁵I-epibatidine binding.
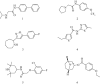
Key results: Functional channels were detected after co-transfection of α6/3, β2 and β3(V273S) subunits, while all other subunit combinations failed to produce agonist-induced responses. Stably expressed α6/3β2β3(V273S)-nAChR pharmacology was unique, and clearly distinct from α4β2-, α3β4-, α7- and α1β1δε-nAChRs. Antagonist potencies in inhibiting α6/3β2β3(V273S) -nAChRs was similar to their binding affinity for rat native α6β2*-nAChRs. Agonist affinities for α6β2*-nAChRs was higher than their potency in activating α6/3β2β3(V273S)-nAChRs, but their relative activities were equivalent. Focussed set screening at α6/3β2β3(V273S)-nAChRs, followed by cross-screening with the other nAChRs, led to the identification of novel α6β2-selective antagonists.
Conclusions and implications: We generated a mammalian cell line stably expressing nAChRs, with pharmacological properties similar to native α6β2*-nAChRs, and used it to identify novel non-peptide, low molecular weight, α6β2-selective antagonists. We also propose a pharmacophore model of α6β2 antagonists, which offers a starting point for the development of new smoking cessation agents.
© 2011 GlaxoSmithKline. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures

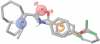
Similar articles
-
Pharmacological and functional comparisons of α6/α3β2β3-nAChRs and α4β2-nAChRs heterologously expressed in the human epithelial SH-EP1 cell line.Acta Pharmacol Sin. 2018 Oct;39(10):1571-1581. doi: 10.1038/aps.2017.209. Epub 2018 May 24. Acta Pharmacol Sin. 2018. PMID: 29795357 Free PMC article.
-
In vitro and in vivo neuronal nicotinic receptor properties of (+)- and (-)-pyrido[3,4]homotropane [(+)- and (-)-PHT]: (+)-PHT is a potent and selective full agonist at α6β2 containing neuronal nicotinic acetylcholine receptors.ACS Chem Neurosci. 2015 Jun 17;6(6):920-6. doi: 10.1021/acschemneuro.5b00077. Epub 2015 Apr 30. ACS Chem Neurosci. 2015. PMID: 25891987 Free PMC article.
-
Characterization of AN6001, a positive allosteric modulator of α6β2-containing nicotinic acetylcholine receptors.Biochem Pharmacol. 2020 Apr;174:113788. doi: 10.1016/j.bcp.2019.113788. Epub 2019 Dec 27. Biochem Pharmacol. 2020. PMID: 31887290
-
Diverse strategies targeting α7 homomeric and α6β2* heteromeric nicotinic acetylcholine receptors for smoking cessation.Ann N Y Acad Sci. 2014 Oct;1327(1):27-45. doi: 10.1111/nyas.12421. Epub 2014 Apr 14. Ann N Y Acad Sci. 2014. PMID: 24730978 Free PMC article. Review.
-
The contribution of agonist and antagonist activities of α4β2* nAChR ligands to smoking cessation efficacy: a quantitative analysis of literature data.Psychopharmacology (Berl). 2018 Sep;235(9):2479-2505. doi: 10.1007/s00213-018-4921-9. Epub 2018 Jul 7. Psychopharmacology (Berl). 2018. PMID: 29980822 Review.
Cited by
-
Therapeutic concentrations of varenicline in the presence of nicotine increase action potential firing in human adrenal chromaffin cells.J Neurochem. 2017 Jan;140(1):37-52. doi: 10.1111/jnc.13883. Epub 2016 Dec 9. J Neurochem. 2017. PMID: 27805736 Free PMC article.
-
Pharmacological and functional comparisons of α6/α3β2β3-nAChRs and α4β2-nAChRs heterologously expressed in the human epithelial SH-EP1 cell line.Acta Pharmacol Sin. 2018 Oct;39(10):1571-1581. doi: 10.1038/aps.2017.209. Epub 2018 May 24. Acta Pharmacol Sin. 2018. PMID: 29795357 Free PMC article.
-
α-Conotoxin PeIA[S9H,V10A,E14N] potently and selectively blocks α6β2β3 versus α6β4 nicotinic acetylcholine receptors.Mol Pharmacol. 2012 Nov;82(5):972-82. doi: 10.1124/mol.112.080853. Epub 2012 Aug 22. Mol Pharmacol. 2012. PMID: 22914547 Free PMC article.
-
Functional alterations by a subgroup of neonicotinoid pesticides in human dopaminergic neurons.Arch Toxicol. 2021 Jun;95(6):2081-2107. doi: 10.1007/s00204-021-03031-1. Epub 2021 Mar 29. Arch Toxicol. 2021. PMID: 33778899 Free PMC article.
-
Acute effects of the imidacloprid metabolite desnitro-imidacloprid on human nACh receptors relevant for neuronal signaling.Arch Toxicol. 2021 Dec;95(12):3695-3716. doi: 10.1007/s00204-021-03168-z. Epub 2021 Oct 10. Arch Toxicol. 2021. PMID: 34628512 Free PMC article.
References
-
- Abad-Zapatero C, Metz JT. Ligand efficiency indices as guideposts for drug discovery. Drug Discov Today. 2005;10:464–469. - PubMed
-
- Andreasen JT, Olsen GM, Wiborg O, Redrobe JP. Antidepressant-like effects of nicotinic acetylcholine receptor antagonists, but not agonists, in the mouse forced swim and mouse tail suspension tests. J Psychopharmacol. 2009;23:797–804. - PubMed
-
- Azam L, McIntosh JM. Effect of novel alpha-conotoxins on nicotine-stimulated [3H] dopamine release from rat striatal synaptosomes. J Pharmacol Exp Ther. 2005;312:231–237. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

