Microglial MAC1 receptor and PI3K are essential in mediating β-amyloid peptide-induced microglial activation and subsequent neurotoxicity
- PMID: 21232086
- PMCID: PMC3027117
- DOI: 10.1186/1742-2094-8-3
Microglial MAC1 receptor and PI3K are essential in mediating β-amyloid peptide-induced microglial activation and subsequent neurotoxicity
Abstract
Background: β-Amyloid peptide (Aβ) is a major protein in the brain associated with Alzheimer's and Parkinson's diseases. The purpose of this study was to investigate the role of macrophage antigen-1 (MAC1) receptor, an integrin scavenger receptor in microglia, and subsequent signaling events in mediating Aβ-induced neurotoxicity. We have previously reported that NADPH oxidase (PHOX) on microglia and superoxide produced by PHOX are critical for Aβ-induced loss of dopaminergic neurons. However, the upstream signaling pathway of superoxide production remains unclear.
Methods: For the in vitro study, mesencephalic neuron-glia cultures and microglia-enriched cultures from mice deficient in the MAC1 receptor (MAC1-/-) and wild type controls were used to investigate the role of MAC1 receptor in Aβ-induced neurotoxicity and the role of phosphoinositide-3 kinase (PI3K) in the signal pathway between MAC1 receptor and PHOX. For the in vivo study, Aβ was injected into the substantia nigra of MAC1-/- mice and wild type mice to confirm the role of MAC1 receptor.
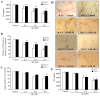
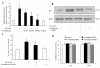
Results: We found that Aβ-induced activation of microglia, activation of PHOX, generation of superoxide and other reactive oxygen species, and loss of dopaminergic neurons were decreased in MAC1-/- cultures compared to MAC1+/+ cultures. In MAC1-/- mice, dopaminergic neuron loss in response to Aβ injection into the substantia nigra was reduced relative to MAC1+/+ mice. Thus, MAC1 receptor-mediated PHOX activation and increased superoxide production are associated with Aβ-induced neurotoxicity. PI3K activation was one downstream step in MAC1 signaling to PHOX and played an important role in Aβ-induced neurotoxicity. In microglia-enriched cultures from MAC1-/- mice, Aβ-induced activation of PI3K (phosphorylation of target proteins and PIP3 production) was reduced relative to MAC1+/+ cultures.
Conclusions: Taken together, our data demonstrate that Aβ activates MAC1 receptor to increase the activity of PI3K, which in turn phosphorylates p47phox, triggers the translocation of cytosolic subunits of PHOX to microglia membrane, increases PHOX activation and the subsequent production of superoxide and causes neurotoxicity.
Figures





References
-
- Jendroska K, Kashiwagi M, Sassoon J, Daniel SE. Amyloid beta-peptide and its relationship with dementia in Lewy body disease. J Neural Transm Suppl. 1997;51:137–144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

