Hyaluronidase recruits mesenchymal-like cells to the lung and ameliorates fibrosis
- PMID: 21232095
- PMCID: PMC3035036
- DOI: 10.1186/1755-1536-4-3
Hyaluronidase recruits mesenchymal-like cells to the lung and ameliorates fibrosis
Abstract
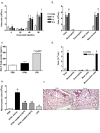
Hyaluronidases (HYALs) comprise a group of enzymes that degrade hyaluronic acid (HA). In this report, we reveal that a single intranasal inoculation of HYAL induces an increase in mononuclear cells within the bronchoalveolar space demonstrating a mesenchymal-like phenotype, expressing stem cell antigen-1 (SCA-1), CD44 and CD73 but not CD34, CD45, CD3, CD4, CD8 or CD19. This influx of mesenchymal stem cell (MSC)-like cells was dependent on leukotriene production within the lung parenchyma. These findings prompted experiments demonstrating that HYAL treatment potently blocked bleomycin-induced lung injury and fibrosis while decreasing transforming growth factor (TGF)-β production and collagen deposition. These data suggest that HYAL is a novel and promising tool to use autologous MSC-like cells in the treatment of pulmonary fibrosis.
Figures


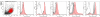


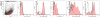
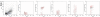

Similar articles
-
Modulation of bleomycin-induced lung fibrosis by pegylated hyaluronidase and dopamine receptor antagonist in mice.PLoS One. 2015 Apr 30;10(4):e0125065. doi: 10.1371/journal.pone.0125065. eCollection 2015. PLoS One. 2015. PMID: 25927611 Free PMC article.
-
Hyaluronidase-loaded PLGA microparticles as a new strategy for the treatment of pulmonary fibrosis.Tissue Eng Part A. 2015 Jan;21(1-2):246-56. doi: 10.1089/ten.TEA.2013.0403. Tissue Eng Part A. 2015. PMID: 25037276
-
CD44-dependent intracellular and extracellular catabolism of hyaluronic acid by hyaluronidase-1 and -2.J Biol Chem. 2007 Feb 23;282(8):5597-607. doi: 10.1074/jbc.M608358200. Epub 2006 Dec 14. J Biol Chem. 2007. PMID: 17170110
-
HYAL-2-WWOX-SMAD4 Signaling in Cell Death and Anticancer Response.Front Cell Dev Biol. 2016 Dec 6;4:141. doi: 10.3389/fcell.2016.00141. eCollection 2016. Front Cell Dev Biol. 2016. PMID: 27999774 Free PMC article. Review.
-
Purification of hyaluronidase as an anticancer agent inhibiting CD44.Biomed Chromatogr. 2020 Jan;34(1):e4709. doi: 10.1002/bmc.4709. Epub 2019 Nov 19. Biomed Chromatogr. 2020. PMID: 31630417 Review.
Cited by
-
Inhibition of endoplasmic reticulum stress is involved in the neuroprotective effect of aFGF in neonatal hypoxic-ischaemic brain injury.Oncotarget. 2017 Apr 29;8(37):60941-60953. doi: 10.18632/oncotarget.17524. eCollection 2017 Sep 22. Oncotarget. 2017. PMID: 28977836 Free PMC article.
-
Hyaluronidase decreases neutrophils infiltration to the inflammatory site.Inflamm Res. 2016 Jul;65(7):533-42. doi: 10.1007/s00011-016-0935-0. Epub 2016 Mar 4. Inflamm Res. 2016. PMID: 26943648
-
Arthropod venom Hyaluronidases: biochemical properties and potential applications in medicine and biotechnology.J Venom Anim Toxins Incl Trop Dis. 2015 Oct 22;21:43. doi: 10.1186/s40409-015-0042-7. eCollection 2015. J Venom Anim Toxins Incl Trop Dis. 2015. PMID: 26500679 Free PMC article.
-
Mechanisms of ivermectin-induced wound healing.BMC Vet Res. 2020 Oct 20;16(1):397. doi: 10.1186/s12917-020-02612-z. BMC Vet Res. 2020. PMID: 33081763 Free PMC article.
-
Efficacy of holmium laser urethrotomy and intralesional injection of Santosh PGI tetra-inject (Triamcinolone, Mitomycin C, Hyaluronidase and N-acetyl cysteine) on the outcome of urethral strictures.Cent European J Urol. 2015;68(4):462-5. doi: 10.5173/ceju.2015.585. Epub 2015 Dec 21. Cent European J Urol. 2015. PMID: 26855803 Free PMC article.
References
-
- Savani RC, Hou G, Liu P, Wang C, Simons E, Grimm PC, Stern R, Greenberg AH, DeLisser HM, Khalil N. A role for hyaluronan in macrophage accumulation and collagen deposition after bleomycin-induced lung injury. Am J Respir Cell Mol Biol. 2000;23:475–484. - PubMed
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

