Brimonidine prevents axonal and somatic degeneration of retinal ganglion cell neurons
- PMID: 21232114
- PMCID: PMC3035592
- DOI: 10.1186/1750-1326-6-4
Brimonidine prevents axonal and somatic degeneration of retinal ganglion cell neurons
Abstract
Background: Brimonidine is a common drug for lowering ocular pressure and may directly protect retinal ganglion cells in glaucoma. The disease involves early loss of retinal ganglion cell transport to brain targets followed by axonal and somatic degeneration. We examined whether brimonidine preserves ganglion cell axonal transport and abates degeneration in rats with elevated ocular pressure induced by laser cauterization of the episcleral veins.
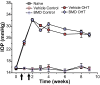
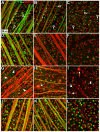
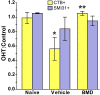
Results: Ocular pressure was elevated unilaterally by 90% for a period of 8 weeks post- cauterization. During this time, brimonidine (1mg/kg/day) or vehicle (phosphate-buffered saline) was delivered systemically and continuously via subcutaneous pump. Animals received bilateral intravitreal injections of fluorescent cholera toxin subunit β (CTB) two days before sacrifice to assess anterograde transport. In retinas from the vehicle group, elevated pressure induced a 44% decrease in the fraction of ganglion cells with intact uptake of CTB and a 14-42% reduction in the number of immuno-labelled ganglion cell bodies, with the worst loss occurring nasally. Elevated pressure also caused a 33% loss of ganglion cell axons in vehicle optic nerves and a 70% decrease in CTB transport to the superior colliculus. Each of these components of ganglion cell degeneration was either prevented or significantly reduced in the brimonidine treatment group.
Conclusions: Continuous and systemic treatment with brimonidine by subcutaneous injection significantly improved retinal ganglion cell survival with exposure to elevated ocular pressure. This effect was most striking in the nasal region of the retina. Brimonidine treatment also preserved ganglion cell axon morphology, sampling density and total number in the optic nerve with elevated pressure. Consistent with improved outcome in the optic projection, brimonidine also significantly reduced the deficits in axonal transport to the superior colliculus associated with elevated ocular pressure. As transport deficits to and from retinal ganglion cell projection targets in the brain are relevant to the progression of glaucoma, the ability of brimonidine to preserve optic nerve axons and active transport suggests its neuroprotective effects are relevant not only at the cell body, but throughout the entire optic projection.
Figures




Similar articles
-
Neuroprotection of retinal ganglion cells by brimonidine in rats with laser-induced chronic ocular hypertension.Invest Ophthalmol Vis Sci. 2001 Nov;42(12):2849-55. Invest Ophthalmol Vis Sci. 2001. PMID: 11687528
-
Neuroprotective effect of alpha(2) agonist (brimonidine) on adult rat retinal ganglion cells after increased intraocular pressure.Brain Res. 2001 Sep 21;913(2):133-9. doi: 10.1016/s0006-8993(01)02759-7. Brain Res. 2001. PMID: 11549376
-
Transient ischemia of the retina results in massive degeneration of the retinotectal projection: long-term neuroprotection with brimonidine.Exp Neurol. 2003 Dec;184(2):767-77. doi: 10.1016/S0014-4886(03)00298-X. Exp Neurol. 2003. PMID: 14769369
-
Assessment of retinal ganglion cell damage in glaucomatous optic neuropathy: Axon transport, injury and soma loss.Exp Eye Res. 2015 Dec;141:111-24. doi: 10.1016/j.exer.2015.06.006. Epub 2015 Jun 9. Exp Eye Res. 2015. PMID: 26070986 Review.
-
Understanding glaucomatous damage: anatomical and functional data from ocular hypertensive rodent retinas.Prog Retin Eye Res. 2012 Jan;31(1):1-27. doi: 10.1016/j.preteyeres.2011.08.001. Epub 2011 Sep 21. Prog Retin Eye Res. 2012. PMID: 21946033 Review.
Cited by
-
Gene expression profiling of transporters in the solute carrier and ATP-binding cassette superfamilies in human eye substructures.Mol Pharm. 2013 Feb 4;10(2):650-63. doi: 10.1021/mp300429e. Epub 2013 Jan 24. Mol Pharm. 2013. PMID: 23268600 Free PMC article.
-
The challenge of regenerative therapies for the optic nerve in glaucoma.Exp Eye Res. 2017 Apr;157:28-33. doi: 10.1016/j.exer.2017.01.007. Epub 2017 Jan 30. Exp Eye Res. 2017. PMID: 28153739 Free PMC article. Review.
-
Non-amyloidogenic effects of α2 adrenergic agonists: implications for brimonidine-mediated neuroprotection.Cell Death Dis. 2016 Dec 8;7(12):e2514. doi: 10.1038/cddis.2016.397. Cell Death Dis. 2016. PMID: 27929541 Free PMC article.
-
The Role of Retinal Ganglion Cell Structure and Function in Glaucoma.Cells. 2023 Dec 8;12(24):2797. doi: 10.3390/cells12242797. Cells. 2023. PMID: 38132117 Free PMC article. Review.
-
Effect of Solution pH on Distribution of Ophthalmically Administered Brimonidine in Posterior Ocular Tissues in Pigmented Rabbits.Ophthalmol Ther. 2019 Jun;8(2):271-277. doi: 10.1007/s40123-019-0180-z. Epub 2019 Mar 20. Ophthalmol Ther. 2019. PMID: 30891685 Free PMC article.
References
-
- Nickells RW. From ocular hypertension to ganglion cell death: a theoretical sequence of events leading to glaucoma. Can J Ophthalmol. 2007;42:278–287. 10.3129/can j ophthalmol.i07-036. - PubMed
-
- Heijl A, Leske MC, Bengtsson B, Hyman L, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–1279. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

