Expression of taste receptors in solitary chemosensory cells of rodent airways
- PMID: 21232137
- PMCID: PMC3031280
- DOI: 10.1186/1471-2466-11-3
Expression of taste receptors in solitary chemosensory cells of rodent airways
Abstract
Background: Chemical irritation of airway mucosa elicits a variety of reflex responses such as coughing, apnea, and laryngeal closure. Inhaled irritants can activate either chemosensitive free nerve endings, laryngeal taste buds or solitary chemosensory cells (SCCs). The SCC population lies in the nasal respiratory epithelium, vomeronasal organ, and larynx, as well as deeper in the airway. The objective of this study is to map the distribution of SCCs within the airways and to determine the elements of the chemosensory transduction cascade expressed in these SCCs.
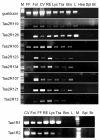
Methods: We utilized a combination of immunohistochemistry and molecular techniques (rtPCR and in situ hybridization) on rats and transgenic mice where the Tas1R3 or TRPM5 promoter drives expression of green fluorescent protein (GFP).
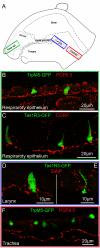
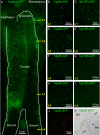
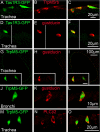
Results: Epithelial SCCs specialized for chemoreception are distributed throughout much of the respiratory tree of rodents. These cells express elements of the taste transduction cascade, including Tas1R and Tas2R receptor molecules, α-gustducin, PLCβ2 and TrpM5. The Tas2R bitter taste receptors are present throughout the entire respiratory tract. In contrast, the Tas1R sweet/umami taste receptors are expressed by numerous SCCs in the nasal cavity, but decrease in prevalence in the trachea, and are absent in the lower airways.
Conclusions: Elements of the taste transduction cascade including taste receptors are expressed by SCCs distributed throughout the airways. In the nasal cavity, SCCs, expressing Tas1R and Tas2R taste receptors, mediate detection of irritants and foreign substances which trigger trigeminally-mediated protective airway reflexes. Lower in the respiratory tract, similar chemosensory cells are not related to the trigeminal nerve but may still trigger local epithelial responses to irritants. In total, SCCs should be considered chemoreceptor cells that help in preventing damage to the respiratory tract caused by inhaled irritants and pathogens.
Figures

References
-
- Smith DV, Hanamori T. Organization of gustatory sensitivities in hamster superior laryngeal nerve fibers. J Neurophysiol. 1991;65(5):1098–1114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

