Complex and rate-dependent beat-to-beat variations in Ca2+ transients of canine Purkinje cells
- PMID: 21232541
- PMCID: PMC3059096
- DOI: 10.1016/j.yjmcc.2010.12.023
Complex and rate-dependent beat-to-beat variations in Ca2+ transients of canine Purkinje cells
Abstract
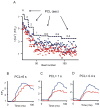
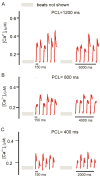
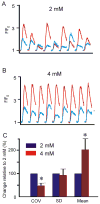
Purkinje fibers play an essential role in transmitting electrical impulses through the heart, but they may also serve as triggers for arrhythmias linked to defective intracellular calcium (Ca(2+)) regulation. Although prior studies have extensively characterized spontaneous Ca(2+) release in nondriven Purkinje cells, little attention has been paid to rate-dependent changes in Ca(2+) transients. Therefore we explored the behaviors of Ca(2+) transients at pacing rates ranging from 0.125 to 3 Hz in single canine Purkinje cells loaded with fluo3 and imaged with a confocal microscope. The experiments uncovered the following novel aspects of Ca(2+) regulation in Purkinje cells: 1) the cells exhibit a negative Ca(2+)-frequency relationship (at 2.5 Hz, Ca(2+) transient amplitude was 66 ± 6% smaller than that at 0.125 Hz); 2) sarcoplasmic reticulum (SR) Ca(2+) release occurs as a propagating wave at very low rates but is localized near the cell membrane at higher rates; 3) SR Ca(2+) load declines modestly (10 ± 5%) with an increase in pacing rate from 0.125 Hz to 2.5 Hz; 4) Ca(2+) transients show considerable beat-to-beat variability, with greater variability occurring at higher pacing rates. Analysis of beat-to-beat variability suggests that it can be accounted for by stochastic triggering of local Ca(2+) release events. Consistent with this hypothesis, an increase in triggering probability caused a decrease in the relative variability. These results offer new insight into how Ca(2+) release is normally regulated in Purkinje cells and provide clues regarding how disruptions in this regulation may lead to deleterious consequences such as arrhythmias.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

